Abstract
Background
Neonates with hypoxic–ischemic encephalopathy (HIE) frequently develop acute kidney injury (AKI). Aminophylline has been shown to reduce severe renal dysfunction in neonates after perinatal asphyxia. However, the effect of aminophylline on renal function in neonates undergoing hypothermia has not been studied.
Methods
A single-center, retrospective chart review of neonates cooled for moderate/severe HIE who received aminophylline for AKI was conducted to assess changes in urine output (UOP) and serum creatinine (SCr). Comparisons were also made to control neonates matched for hours of life who were cooled but unexposed to aminophylline.
Results
Sixteen neonates cooled for HIE received aminophylline starting at 25 ± 14 h of life. Within 12 h of starting aminophylline, UOP increased by 2.6 ± 1.9 mL/kg/h. SCr declined by 0.4 ± 0.2 mg/dL in survivors over the first 4 days. When compared to control neonates, UOP increase was greater in the aminophylline group (p < 0.001). SCr declined in survivors in both groups, although baseline SCr was higher in the aminophylline group.
Conclusions
Aminophylline use in neonates with HIE undergoing hypothermia was associated with an increase in UOP and a decline in SCr. A randomized trial will be needed to establish a potential renal protective role of aminophylline.
Impact
-
The renal protective effect of aminophylline in neonates with HIE has not yet been studied in the context of therapeutic hypothermia.
-
Aminophylline exposure in neonates cooled for HIE was associated with increased UOP and a similar decline in SCr when compared to control infants unexposed to aminophylline.
-
Improved renal function after receiving aminophylline in this observational cohort study suggests the need for future randomized trials to establish the potential benefit of aminophylline in the HIE population undergoing hypothermia.
Similar content being viewed by others
Introduction
Neonates with hypoxic–ischemic encephalopathy (HIE) are at high risk of acute kidney injury (AKI) with several reports of an incidence as high as 40%.1,2,3 Antenatal and postnatal hypoxia, hypotension, and use of nephrotoxic medications may contribute to ongoing renal insult. The occurrence of AKI in neonates with HIE is an independent risk factor for adverse outcomes, including prolonged mechanical ventilation, prolonged length of hospital stay, injury on magnetic resonance imaging (MRI), and increased risk of long-term neurodevelopmental impairment.1,4,5,6 While therapeutic hypothermia has been shown to improve neurodevelopmental outcomes in neonates with birth asphyxia,7 there has been limited research into pharmacotherapies that may reduce AKI in this vulnerable population.
Theophylline and aminophylline (the ethylenediamine salt formulation of theophylline and same active molecule) are potentially targeted therapeutic agents for AKI through action as non-selective adenosine receptor antagonists. During hypoxia/ischemia, intrarenal vasoconstriction occurs as a consequence of higher adenosine levels with a subsequent reduction in renal blood flow and fall in glomerular filtration rate (GFR) and filtration fraction.8 In newborn animal models, administration of low-dose theophylline prevented hypoxemia-associated reductions in GFR and filtration fraction.8,9 Theophylline or aminophylline for renal protection has been studied in infants with congenital heart disease after cardiac surgery,10,11 neonates on extracorporeal membrane oxygenation support,12 and preterm infants with apnea of prematurity,13,14 with reports of augmented urine output (UOP), improved creatinine clearance, and no adverse effects. However, the physiologic effect of theophylline on renal function in the HIE population remains unclear. Neonates being cooled for HIE with AKI have higher renal saturation measures compared to those without AKI,15 possibly reflecting decreased extraction of oxygen by an injured kidney. Theophylline may increase renal blood flow post-hypoxic insult and lead to improved renal saturation measures.
In single-center, randomized controlled trials, a single dose of theophylline shortly after birth has also been shown to improve UOP and reduce the incidence of severe renal dysfunction in neonates with severe perinatal asphyxia.16,17,18,19,20 Based on available evidence, the 2011 Kidney Disease Improving Global Outcomes (KDIGO) guidelines suggest a single dose of theophylline to be considered for prevention of AKI in the HIE population (moderate quality of evidence).21 However, all theophylline clinical studies to date were conducted before the widespread use of therapeutic hypothermia (whole-body cooling) for HIE, and the therapeutic benefit of theophylline (or aminophylline) in the context of hypothermia remains unknown.
Guided by its underlying mechanism of action and previous clinical studies in asphyxiated neonates, our center has been using aminophylline as a renal protective management strategy for several years in neonates with HIE undergoing hypothermia who develop low UOP and/or rising serum creatinine (SCr). We first report our experience on the use of aminophylline during clinical care in a cohort of neonates being cooled for HIE with a descriptive analysis of the impact of treatment on clinical biomarkers of renal function, including UOP, SCr, and renal saturation. Second, we compared the time-course of clinical biomarkers in those who received aminophylline with a time-matched control group of neonates with HIE undergoing hypothermia, but unexposed to aminophylline. We hypothesized that treatment with aminophylline in neonates with HIE undergoing hypothermia would be associated with a greater increase in UOP and larger decrease in SCr compared to those who did not receive aminophylline.
Methods
Term neonates with moderate or severe HIE undergoing therapeutic hypothermia at a tertiary care neonatal intensive care unit between 1 January 2014 and 31 December 2017 were assessed for a descriptive study of neonates exposed to aminophylline during clinical care and also a matched observational cohort study comparing neonates exposed and unexposed to aminophylline. For the matched cohort study, neonates who received aminophylline during clinical care served as the aminophylline group. A control group, matched 1:1 based on nearest time of admission, was identified from the population of neonates with HIE undergoing hypothermia who did not receive aminophylline. Aminophylline was initiated per clinician discretion for low UOP, rising SCr and/or other concern for AKI with an intravenous loading dose of 5 mg/kg (equivalent to 3.9 mg/kg theophylline), followed by maintenance dose of 1.8 mg/kg (equivalent to 1.4 mg/kg theophylline) every 6 h. Theophylline levels were monitored as part of clinical care and adjusted to maintain a level of 5–7 mg/L. Aminophylline was discontinued per discretion of the care team. Whole-body cooling per clinical protocol was achieved using a CritiCool thermoregulation system (Belmont Medical Technologies, Billerica, MA) to maintain esophageal temperature at 33.5 °C for 72 h, followed by rewarming to 37 °C by 0.5 °C/h. All neonates underwent monitoring with video electroencephalography during cooling and rewarming as per clinical routine. This study was approved by the Stanford Institutional Review Board with waiver of consent.
Neonatal characteristics and outcome data were obtained from the electronic medical record. All SCr levels during the birth hospitalization and UOP for 12 h before and 72 h after aminophylline were recorded. Near-infrared spectroscopy (NIRS) monitoring of cerebral and renal saturation levels (Csat and Rsat) was used routinely as per institutional standard of care during hypothermia and through the rewarming period (INVOS 5100C, Medtronic, Minnesota). In those neonates with NIRS data available, the Rsat and Csat for 12 h before and 72 h after aminophylline administration were collected. AKI was determined using criteria from the AWAKEN study, specifically the KDIGO criteria modified for neonates and defining AKI as a rise in SCr of 0.3 mg/dL or 50% from the lowest previous value and/or UOP <1 mL/kg/h averaged over 24 h after the first 24 h of life.22
Statistical analyses
For the descriptive study of neonates receiving aminophylline, the closest SCr measured before aminophylline start served as the baseline and was compared to SCr over the next 4 days after aminophylline start. UOP (mL/kg/h) over the 12 h before aminophylline start served as the baseline and was compared to UOP over the next 72 h in 12 h blocks of time for the first 24 h, followed by 24 h blocks of time. For Rsat and Csat, the mean value over the 12 h before aminophylline start was compared to the mean value over the 12 h after aminophylline. Changes in UOP, SCr, Rsat, and Csat before and after aminophylline start were compared using repeated-measures analysis of variance (ANOVA) (Stata V13, StataCorp, College Station, TX). For neonates who died prior to the end of the study period, the last observation carried forward was used. Data are reported as mean ± standard deviation or counts (%), unless otherwise specified.
Given the large changes in renal physiology, blood flow, and function that occur over the first days of life after birth, it was important to compare any changes in SCr, UOP, Rsat, and Csat measures after aminophylline start with a control group of neonates cooled for HIE evaluated over similar hours of life. Therefore, each patient in the aminophylline group was matched 1:1 with a control patient, and the hours of life when aminophylline was given to the aminophylline patient served as the reference time = 0 h for both patients in a pair. This created 16 pairs. In the unexposed control group, the same outcome measures were calculated as for the aminophylline group for pre and post comparisons. Fisher’s exact, Mann–Whitney U test, or Student’s t test compared categorical, ordinal, and continuous characteristics and outcomes between neonates exposed to aminophylline and unexposed controls (R environment, version 3.5.3). Repeated-measures ANOVA was used to assess differences between groups in SCr, UOP, Rsat, and Csat. Given a distinct response of UOP and SCr in those who survived compared to those who died, analyses were also performed comparing three groups: aminophylline survivors, aminophylline non-survivors, and control. Significance was set at p < 0.05 for all analyses.
Results
Neonates receiving aminophylline
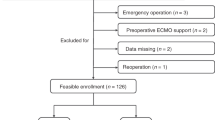
Of 97 neonates with HIE undergoing therapeutic hypothermia during the study period, 16 neonates received aminophylline. Clinical characteristics are shown in Table 1. On average, aminophylline was started at 25.2 ± 13.5 h of life and continued for a mean of 2.6 ± 2.2 days. In 13 of 16 neonates, theophylline drug levels were measured during the first day of therapy with a mean concentration of 6.8 ± 1.2 mg/L (range 5.3–8.4 mg/L). In eight neonates, theophylline levels were repeated on the second or third day of therapy with an increase to a mean concentration of 11.3 ± 2.1 mg/L, and seven neonates had a theophylline concentration >10 mg/L. All were receiving 1.8 mg/kg every 6 h at the time of drug level measurement.
Clinical outcomes after aminophylline
Four neonates who received aminophylline died after withdrawal of intensive care support for poor neurologic prognosis and multiorgan failure (mean age of 67 h, range 32–95 h). Eleven patients developed clinical or electrographic seizures, with a mean age at detection of 41.4 h of life (range 2.7–176.6). Five neonates (45%) first demonstrated seizures prior to the administration of aminophylline, and two (18%) first demonstrated seizures after aminophylline was discontinued (16 and 122 h after discontinuation of medication). No adverse events related to aminophylline administration were described.
Clinical biomarkers of renal function
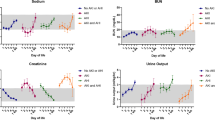
UOP response to aminophylline by survival status is shown in Fig. 1. One neonate started therapy within 7 h of birth, and UOP data were not available prior to the start, leaving 15 subjects for UOP analysis. In the 12 h after aminophylline start, UOP significantly increased from 0.7 ± 0.5 mL/kg/h to 3.2 ± 2.1 mL/kg/h (change of 2.6 ± 1.9 mL/kg/h; p < 0.001). This increase in UOP was sustained over the 72 h period of study (p < 0.001 for all time points). Compared to non-survivors (n = 4), neonates who survived (n = 12) had a similar pre-aminophylline UOP (p = 0.7), but a higher UOP for all time points after aminophylline start (p < 0.01).
Neonates receiving aminophylline (n = 12 survivors and n = 4 non-survivors) had lower urine output (UOP) during the 12-h period prior to the start of aminophylline compared to an age-matched time period in control patients (n = 16) (*p < 0.05 vs. control at time point). A significant increase in UOP from baseline was seen after aminophylline treatment in survivors at all time points (#p < 0.001 vs. “pre” time point) and in control patients at 24 and 72 h (+p < 0.05 vs. “pre” time point). UOP was significantly higher during the first 12 h after aminophylline start in survivors compared to control patients (*p < 0.05 vs. control at time point), but no differences in UOP between these two groups were seen at later time points. UOP in non-survivors who received aminophylline did not increase significantly over the study period.
Changes in SCr after aminophylline by survival status are shown in Fig. 2. Two neonates died within 2 days of aminophylline therapy, and SCr was not available beyond 24 h after aminophylline. Two neonates continued to have a rising SCr up to 72 h after aminophylline was started; both of these infants also died. In comparison, neonates who survived demonstrated a significant decline in SCr by −0.2 ± 0.3 mg/dL from baseline by 48 h after the start of aminophylline (p < 0.01) and by −0.4 ± 0.2 mg/dL from baseline by 96 h after aminophylline (p < 0.001).
Serum creatinine (SCr) significantly decreased from baseline by 48 h after aminophylline in survivors (n = 12) and by 24 h during an age-matched time period of life in control patients (n = 16, #p < 0.05 vs. “pre” time point within each group). SCr remained significantly higher at 24, 48, and 72 h in survivors compared to control patients (*p < 0.05 vs. control). Non-survivors (n = 4) in the aminophylline group had a rising SCr over the study period prior to withdrawal of intensive care support.
Regarding NIRS measures, no significant changes in Rsat or Csat were detected during the 12 h period after the start of aminophylline therapy. However, there was a positive correlation between Rsat prior to aminophylline exposure and increased UOP after the start of therapy (Fig. 3).
Aminophylline vs. control group comparison
From the original 76 neonates who did not receive aminophylline while being cooled for HIE, 16 neonates with sufficient NIRS, SCr, and UOP data were selected as the control cohort matched on nearest time of admission. Demographic and perinatal characteristics of the aminophylline and control groups are shown in Table 1. Patients were similar except that the aminophylline group was of slightly lower gestational age (38 ± 2 vs. 39 ± 2 weeks, p = 0.03), more often delivered by Cesarean section (94% vs. 44%, p = 0.04), and had lower 5-min Apgar scores (median 4 [interquartile range (IQR) 2–5] vs. 6 [IQR 4–7], p = 0.01). There was no statistically significant difference between aminophylline and control groups for the concomitant use of other drugs potentially affecting renal function, although neonates receiving aminophylline more often required pressor support (9 in aminophylline group vs. 5 in control group). There was no association between pressor use and change in UOP (p = 0.79).
Clinical outcomes and discharge measures of comparative cohort
Six of 16 patients in the aminophylline group met the criteria for AKI by the modified KDIGO criteria compared to none in the unexposed group (38% vs. 0%, p = 0.02). Three neonates met the AKI criteria based on SCr only, one neonate based on UOP only, and two neonates based on both. The remaining 26 patients in the cohort did not meet any criteria for AKI.
Although not all statistically significant, neonates exposed to aminophylline also had other markers of poorer clinical outcomes compared to controls, including increased death (25% vs. 6%, p = 0.33), increased moderate to severe MRI abnormalities (58% vs. 13%, p = 0.02),23 and an increased rate of clinical or electrographic seizures (69% vs. 44%, p = 0.15) (Table 1). While the four deaths in the aminophylline group occurred within 96 h of life due to multiorgan failure, the only patient death in the control group occurred at 20 days of age after prolonged respiratory failure and seizures. At the time of hospital discharge (Table 1), there was no difference in SCr levels (0.38 ± 0.14 vs. 0.36 ± 0.09 mg/dL) or systolic blood pressure percentiles for postmenstrual age (37%ile vs. 36%ile)24 between groups, although those receiving aminophylline were more likely to have a longer duration of hospitalization (21 ± 11 vs. 15 ± 7 days). No infants had neonatal hypertension (blood pressure >95%ile for postmenstrual age).
UOP, SCr, and NIRS measures of comparative cohort
Clinical biomarkers of renal function were compared between the aminophylline and control groups. Infants receiving aminophylline had a lower UOP during the 12-h period prior to the start of therapy compared to the control group during an age-matched 12-h period (0.7 ± 0.5 vs. 1.9 ± 1.1 mL/kg/h, p = 0.01). UOP was <1 mL/kg/h for 12 neonates in the aminophylline group and three neonates in the control group during this pre-aminophylline baseline period. The mean increase in UOP from baseline in the aminophylline group during the 12 h period after the start of therapy was 2.6 ± 1.9 mL/kg/h (p < 0.001) compared to an increase from baseline of 0.6 ± 1.2 mL/kg/h in the control group (p = 0.16). Eleven of 15 neonates (73%) receiving aminophylline had >1 mL/kg/h increase in UOP after aminophylline compared to 6 of 16 (38%) controls over the same age-matched period (p = 0.07). By 12 h after aminophylline start and continuing for the remainder of the 72-h period of study, there were no longer significant differences in UOP between aminophylline and control groups. UOP in controls is compared to aminophylline survivors and aminophylline non-survivors in Fig. 1.
Infants receiving aminophylline had a higher SCr prior to the start of therapy compared to the control group at an age-matched day of life (1.0 ± 0.3 vs. 0.8 ± 0.2 mg/dL, p < 0.05). In aminophylline survivors (n = 12) and control patients (n = 16), SCr decreased from baseline over the 96-h study period by 0.3 ± 0.2 mg/dL (p < 0.001) and 0.4 ± 0.2 mg/dL (p < 0.001), respectively (Fig. 2). The one neonate in the control group who died had a declining SCr after birth. The mean SCr remained significantly higher in the aminophylline survivors compared to the control group at all time points except 96 h.
NIRS measures of Rsat and Csat were not significantly different between aminophylline and control groups during the period of study (Table 2). The positive relationship between baseline Rsat and UOP seen in the aminophylline group was not seen in the control group (data not shown).
Discussion
In this observational cohort of neonates with HIE undergoing therapeutic hypothermia, we aimed to describe the impact of receiving aminophylline during clinical care on biomarkers of renal function. We secondly compared these patients receiving aminophylline to a time-matched control group not exposed to aminophylline. We found that aminophylline was associated with a rapid and robust increase in UOP and a slow decline in SCr in survivors. In contrast, non-survivors exposed to aminophylline did not demonstrate a significant increase in UOP and continued to have rising SCr after aminophylline start. A control group of cooled HIE patients not exposed to aminophylline started with a higher baseline UOP, but UOP increased in aminophylline survivors to be similar to or greater than control patients within 12 h of aminophylline exposure. Control patients had a lower baseline SCr, which declined over the first days of life. However, SCr was similar between aminophylline survivors and control patients by the end of the study period and at hospital discharge. NIRS measures of Csat and Rsat were not impacted by aminophylline, although a higher Rsat at baseline was associated with a greater UOP response to aminophylline. A randomized clinical trial of aminophylline for renal protection in the HIE population will be necessary before its widespread use in clinical care.
The current study represents the first published experience of theophylline, here given as its salt formulation aminophylline, in neonates with HIE undergoing hypothermia. Similar to randomized clinical trials of theophylline without the use of hypothermia in neonates with HIE, theophylline exposure increased UOP and was associated with a decline in SCr. Given the observational nature of this study, we were unable to examine the impact of theophylline exposure on the risk of AKI due to confounding by indication, where only neonates with worsening renal dysfunction were started on aminophylline. For example, of the six neonates who met the AWAKEN criteria for AKI, four were diagnosed at the time of aminophylline start. The aminophylline group was also more clinically ill at baseline with poorer outcomes (higher rates of seizures, MRI abnormalities, length of stay, and death) compared to the control group. These neonates may already have developed an advanced degree of kidney injury no longer responsive to the benefits of aminophylline. Moreover, aminophylline was initiated at a mean age of 25.2 ± 13.5 h of life. In contrast, in the clinical trials to date demonstrating a lower risk of AKI with theophylline (relative risk 0.40 [0.30–0.54]) in the HIE population, neonates were randomized at birth to treatment within the first hour of life.16,17,18,19,20 Prevention or amelioration of AKI may be most effective when theophylline exposure occurs soon after birth (prior to 24 h of life or even sooner), similar to the benefit of cooling by 6 h of life to reduce the rate of death or moderate or severe neurodevelopmental disability.
The optimal theophylline exposure needed for prevention or treatment of AKI in neonates with HIE is not known. Only one randomized controlled trial of theophylline in neonates with HIE measured drug levels during treatment with a mean theophylline concentration of 12.7 g/mL (range: 7.5–18.9 g/mL) at 36–48 h of life.16 In this study, the dose of theophylline was 8 mg/kg (=10 mg/kg aminophylline). Similar outcomes were found when lower theophylline doses of 5 mg/kg (6.3 mg/kg) were used and indicate lower exposures are effective.17,19,20 In our study, theophylline concentrations of 5.3–8.4 mg/L during the first day of therapy resulted in clinically significant increases in UOP and declines in SCr. Within this concentration range, no correlation between theophylline concentration and UOP were observed (R2 = 0.1; data not shown) suggesting no additional benefit of higher exposures. Our findings agree with prior in vitro studies, which have shown that theophylline concentrations of 1–2 mg/L are adequate to inhibit adenosine receptors (subtypes A1 and A2) involved in renal hemodynamics.25,26,27 In newborn rabbits, theophylline concentrations <0.7 mg/L prevented hypoxemia-induced changes in GFR and renal blood flow.8 Theophylline concentrations ranging from 1 to 10 mg/L have been associated with improvement in UOP or SCr in clinical studies in adults, children, and neonates.13,28,29,30 Taken together, current evidence suggests that theophylline concentrations of 4–8 mg/L are a reasonable target for renal protective indications. These concentrations are lower than needed for theophylline’s bronchodilator effects (10–20 mg/L) and will also minimize toxicity concerns seen at higher concentration (>20 mg/L).
Given the narrow therapeutic index of theophylline, understanding the pharmacokinetics of theophylline will be critical to advance its therapeutic use and support a safe and effective dosing strategy. The dose of aminophylline used in our population was a 5 mg/kg (=4 mg/kg of theophylline) load, followed by maintenance doses of 1.8 mg/kg (=1.4 mg/kg of theophylline) every 6 h. While exposures during the first day of treatment were appropriate, this dosing regimen resulted in ongoing accumulation with seven of eight patients having theophylline concentrations of >10 mg/L on the second or third day of treatment. The pharmacokinetics of medications in neonates with HIE undergoing hypothermia are known to be unique, and reduced drug clearance and need for lower doses of several medications has been described.31,32,33 To guide the development of an individualized dosing strategy of theophylline for neonates with HIE undergoing hypothermia, our group has recently conducted a clinical pharmacokinetic study of theophylline (given as aminophylline) and analysis is currently underway.
Neonates with higher renal saturations at baseline had larger increases in UOP after aminophylline in our study. This finding parallels our previous research demonstrating increased renal saturations in those infants with AKI,15 possibly identifying a high-risk population that would most benefit from aminophylline. Rsat measures did not significantly increase in the aminophylline group, despite an expected physiologic increase in renal blood flow with adenosine receptor inhibition. The expected increase in Rsat after aminophylline may have been blunted by hypothermia-induced renal vasoconstriction. Alternatively, renal blood flow (and oxygen delivery) may have increased with an equivalent increase in metabolic activity of the renal parenchyma. Increased GFR and changes in tubular reabsorption after aminophylline may have contributed to improved UOP, but these mechanisms were not detectable using non-invasive monitoring of tissue saturation.
The SCr response to aminophylline was less clear from this observational study. Both aminophylline-exposed survivors and control neonates had a significant decline in SCr from baseline over the 96-h study period, which is similar to the expected trajectory over time after birth.3 However, it is unclear if the patients receiving aminophylline would otherwise have had a rise in their SCr or a slower rate of decline. For example, in their cohort of asphyxiated newborns treated with hypothermia, Selewski et al.1 found that those who developed AKI had a rising SCr in the first 72 h of life, no decline from baseline until after 7 days, and an elevated SCr at the time of discharge.1 Additional investigation is needed to target those individuals most likely to respond to aminophylline.
Similar to aminophylline and theophylline, other methylxanthines, including caffeine, may also be effective therapeutic treatments through adenosine receptor inhibition. Caffeine is a compelling drug to investigate given its longstanding and widespread use in the preterm NICU population for apnea and neuroprotection, as well as its favorable safety profile. Moreover, retrospective studies suggest a renal protective effect in the preterm population who received caffeine for apnea.34,35 However, before caffeine can be advanced for renal protection, further understanding is needed. In animal models, the effects of theophylline and caffeine on renal hemodynamics were not the same,36 and caffeine has not been studied in models of hypoxemia to evaluate its effect on kidney function in this setting. More importantly, the use of caffeine in neonates with HIE undergoing hypothermia has not yet been established and no clinical studies have examined it use in this population. At this time, only theophylline has been endorsed in KDIGO clinical practice guidelines as a potential treatment option in neonates with HIE to prevent AKI (moderate level of evidence based on available literature in 2011).21
As the population of infants with HIE are at high risk for brain injury and neurodevelopmental impairment, an ideal renal protective agent would not have adverse effects on the brain. Our study demonstrated no change in cerebral saturation for patients receiving aminophylline. This finding is consistent with animal and newborn studies showing transient minimal effects of aminophylline on cerebral blood flow.37,38 Although MRI abnormalities were more significant in the aminophylline-exposed group, it is known that neonates with brain injury after birth asphyxia are also more likely to have multiorgan dysfunction, including AKI.4,39 Theophylline and other methylxanthines have been associated with an increased risk of central nervous system excitation and seizures at high doses, particularly with theophylline concentrations over 100 mg/L or with chronic use >30 mg/L.40,41,42 However, the clinical trials in HIE patients using theophylline at a standard dose of 5 mg/kg did not demonstrate any difference in seizure burden.16,17,19,20 Our study showed that seizures occurred in 11 infants exposed to aminophylline (69%) compared to seven unexposed infants (44%). However, in the aminophylline-exposed group, almost half of the infants had their seizures first detected prior to administration of aminophylline. Seizures in HIE patients typically occur in the first 24–48 h of life,43,44 and in our study, it most likely reflects the more severe degree of encephalopathy in the population receiving aminophylline. Moreover, theophylline levels in those infants with seizures were all within 5.4–8.4 mg/L range, making theophylline toxicity an unlikely cause of lowered seizure threshold. Nonetheless, further safety data of theophylline exposure in the HIE population in well-controlled trials is needed.
This study is limited by lack of long-term renal function data and incidence of chronic kidney disease. Although SCr and blood pressure measures at the time of hospital discharge were not abnormal in our cohort, follow-up data were not available to assess possible longer-term complications. The literature remains sparse with regards to which infants with HIE progress from AKI to chronic kidney disease.45,46,47 Additional measures, including urine biomarkers or imaging studies, may provide further insight into the potential protective effect of aminophylline. We also caution that our direct comparison of an observational HIE control group with HIE patients started on aminophylline for clinical care is limited given the inherent differences in illness severity, which impact biomarkers of kidney function and clinical outcomes. Moreover, the aminophylline-exposed group had increased severity of perinatal asphyxia and HIE as demonstrated by significantly lower 5-min Apgar score compared to the control group. Other investigators have demonstrated that severity of encephalopathy predicts renal failure even more than SCr,48 again highlighting the inherent difficulties of an observational study of aminophylline exposure. A randomized clinical trial of aminophylline in the HIE population undergoing cooling is therefore necessary to establish the potential therapeutic benefit of aminophylline, including measures of longer-term kidney function for prevention of chronic renal dysfunction.
Conclusions
Aminophylline exposure in neonates with HIE undergoing therapeutic hypothermia was associated with increased UOP and a decline in SCr in survivors. Control neonates not exposed to aminophylline exhibited a smaller increase in UOP over time and had a similar decline in SCr after starting from a lower baseline. No adverse effects were associated with aminophylline administration. Future investigations of aminophylline should focus on optimal dosing strategies, importance of timing and duration of exposure, long-term markers of kidney function, and identification of sub-groups most likely to benefit. This knowledge will inform the design of a clinical efficacy trial of aminophylline as a renal protective treatment in a population of neonates undergoing hypothermia for HIE.
References
Selewski, D. T., Jordan, B. K., Askenazi, D. J., Dechert, R. E. & Sarkar, S. Acute kidney injury in asphyxiated newborns treated with therapeutic hypothermia. J. Pediatr. 162, 725–729.e1 (2013).
Kirkley, M. J. et al. Acute kidney injury in neonatal encephalopathy: an evaluation of the AWAKEN database. Pediatr. Nephrol. 34, 169–176 (2019).
Gupta, C., Massaro, A. N. & Ray, P. E. A new approach to define acute kidney injury in term newborns with hypoxic ischemic encephalopathy. Pediatr. Nephrol. 31, 1167–1178 (2016).
Sarkar, S. et al. Relationship between acute kidney injury and brain MRI findings in asphyxiated newborns after therapeutic hypothermia. Pediatr. Res. 75, 431–435 (2014).
Cavallin, F. et al. Prognostic role of acute kidney injury on long-term outcome in infants with hypoxic–ischemic encephalopathy. Pediatr. Nephrol. 35, 477–483 (2020).
Stoops, C. et al. The association of intraventricular hemorrhage and acute kidney injury in premature infants from the Assessment of the Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) Study. Neonatology 116, 321–330 (2019).
Shankaran, S. et al. Whole-body hypothermia for neonates with hypoxic–ischemic encephalopathy. N. Engl. J. Med. 353, 1574–1584 (2005).
Gouyon, J. B. & Guignard, J. P. Theophylline prevents the hypoxemia-induced renal hemodynamic changes in rabbits. Kidney Int. 33, 1078–1083 (1988).
Osswald, H., Gleiter, C. & Mühlbauer, B. Therapeutic use of theophylline to antagonize renal effects of adenosine. Clin. Nephrol. 43(Suppl. 1), S33–37 (1995).
Axelrod, D. M., Sutherland, S. M., Anglemyer, A., Grimm, P. C. & Roth, S. J. A double-blinded, randomized, placebo-controlled clinical trial of aminophylline to prevent acute kidney injury in children following congenital heart surgery with cardiopulmonary bypass. Pediatr. Crit. Care Med. 17, 135–143 (2016).
Axelrod, D. M. et al. Initial experience using aminophylline to improve renal dysfunction in the pediatric cardiovascular ICU. Pediatr. Crit. Care Med. 15, 21–27 (2014).
Lochan, S. R. et al. Coadministration of theophylline enhances diuretic response to furosemide in infants during extracorporeal membrane oxygenation: a randomized controlled pilot study. J. Pediatr. 133, 86–89 (1998).
Mazkereth, R. et al. Effects of theophylline on renal function in premature infants. Am. J. Perinatol. 14, 45–49 (1997).
Cattarelli, D. et al. A randomised, double blind, placebo controlled trial of the effect of theophylline in prevention of vasomotor nephropathy in very preterm neonates with respiratory distress syndrome. Arch. Dis. Child. Fetal Neonatal Ed. 91, F80–84 (2006).
Chock, V. Y., Frymoyer, A., Yeh, C. G. & Van Meurs, K. P. Renal saturation and acute kidney injury in neonates with hypoxic–ischemic encephalopathy undergoing therapeutic hypothermia. J. Pediatr. 200, 232–239.e1 (2018).
Jenik, A. G. et al. A randomized, double-blind, placebo-controlled trial of the effects of prophylactic theophylline on renal function in term neonates with perinatal asphyxia. Pediatrics 105, E45 (2000).
Bakr, A. F. Prophylactic theophylline to prevent renal dysfunction in newborns exposed to perinatal asphyxia—a study in a developing country. Pediatr. Nephrol. 20, 1249–1252 (2005).
Bhat, M. A., Shah, Z. A., Makhdoomi, M. S. & Mufti, M. H. Theophylline for renal function in term neonates with perinatal asphyxia: a randomized, placebo-controlled trial. J. Pediatr. 149, 180–184 (2006).
Eslami, Z., Shajari, A., Kheirandish, M. & Heidary, A. Theophylline for prevention of kidney dysfunction in neonates with severe asphyxia. Iran. J. Kidney Dis. 3, 222–226 (2009).
Raina, A., Pandita, A., Harish, R., Yachha, M. & Jamwal, A. Treating perinatal asphyxia with theophylline at birth helps to reduce the severity of renal dysfunction in term neonates. Acta Paediatr. 105, e448–451 (2016).
Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 120, c179–184 (2012).
Jetton, J. G. et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc. Health 1, 184–194 (2017).
Barkovich, A. J. et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. Am. J. Neuroradiol. 19, 143–149 (1998).
Zubrow, A. B., Hulman, S., Kushner, H. & Falkner, B. Determinants of blood pressure in infants admitted to neonatal intensive care units: a prospective multicenter study. Philadelphia Neonatal Blood Pressure Study Group. J. Perinatol. 15, 470–479 (1995).
Kim, S.-A. et al. Structure-activity relationships at human and rat A2B adenosine receptors of xanthine derivatives substituted at the 1-, 3-, 7-, and 8-positions. J. Med. Chem. 45, 2131–2138 (2002).
Klotz, K. N. et al. Comparative pharmacology of human adenosine receptor subtypes—characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch. Pharmacol. 357, 1–9 (1998).
Jacobson, K. A., Ijzerman, A. P. & Linden, J. 1,3-Dialkylxanthine derivatives having high potency as antagonists at human A2B adenosine receptors. Drug Dev. Res. 47, 45–53 (1999).
Tamburro, R. F. et al. A prospective assessment of the effect of aminophylline therapy on urine output and inflammation in critically ill children. Front. Pediatr. 2, 59 (2014).
Lynch, B. A. et al. Low-dose aminophylline for the treatment of neonatal non-oliguric renal failure-case series and review of the literature. J. Pediatr. Pharm. Ther. 13, 80–87 (2008).
Dai, B. et al. Effect of theophylline on prevention of contrast-induced acute kidney injury: a meta-analysis of randomized controlled trials. Am. J. Kidney Dis. 60, 360–370 (2012).
Welzing, L. et al. Disposition of midazolam in asphyxiated neonates receiving therapeutic hypothermia—a pilot study. Klin. Padiatr. 225, 398–404 (2013).
Frymoyer, A. et al. Decreased morphine clearance in neonates with hypoxic–ischemic encephalopathy receiving hypothermia. J. Clin. Pharm. 57, 64–76 (2017).
Frymoyer, A., Meng, L., Bonifacio, S. L., Verotta, D. & Guglielmo, B. J. Gentamicin pharmacokinetics and dosing in neonates with hypoxic ischemic encephalopathy receiving hypothermia. Pharmacotherapy 33, 718–726 (2013).
Harer, M. W. et al. Association between early caffeine citrate administration and risk of acute kidney injury in preterm neonates: results from the AWAKEN study. JAMA Pediatr. 172, e180322 (2018).
Carmody, J. B., Harer, M. W., Denotti, A. R., Swanson, J. R. & Charlton, J. R. Caffeine exposure and risk of acute kidney injury in a retrospective cohort of very low birth weight neonates. J. Pediatr. 172, 63–68.e1 (2016).
Gouyon, J. B. & Guignard, J. P. Renal effects of theophylline and caffeine in newborn rabbits. Pediatr. Res. 21, 615–618 (1987).
Stonestreet, B. S., Nowicki, P. T., Hansen, N. B., Petit, R. & Oh, W. Effect of aminophylline on brain blood flow in the newborn piglet. Dev. Pharm. Ther. 6, 248–258 (1983).
Dani, C. et al. Brain hemodynamic changes in preterm infants after maintenance dose caffeine and aminophylline treatment. Biol. Neonate 78, 27–32 (2000).
Gupta, B. D., Sharma, P., Bagla, J., Parakh, M. & Soni, J. P. Renal failure in asphyxiated neonates. Indian Pediatr. 42, 928–934 (2005).
Clancy, R. R., Kaplan, K. M., Baumgart, S. & Rosenberry, K. R. Neonatal theophylline neurotoxicity. Clin. Pediatr. (Philos.) 24, 168–170 (1985).
Aranda, J. V., Chemtob, S., Laudignon, N. & Sasyniuk, B. I. Pharmacologic effects of theophylline in the newborn. J. Allergy Clin. Immunol. 78, 773–780 (1986).
Hospira, Inc. FDA Package Insert Aminophylline (Aminophylline Injection, Solution) (2009).
Nash, K. B. et al. Video-EEG monitoring in newborns with hypoxic–ischemic encephalopathy treated with hypothermia. Neurology 76, 556–562 (2011).
Glass, H. C. et al. Risk factors for EEG seizures in neonates treated with hypothermia: a multicenter cohort study. Neurology 82, 1239–1244 (2014).
Harer, M. W., Pope, C. F., Conaway, M. R. & Charlton, J. R. Follow-up of Acute kidney injury in Neonates during Childhood Years (FANCY): a prospective cohort study. Pediatr. Nephrol. 32, 1067–1076 (2017).
Mammen, C. et al. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am. J. Kidney Dis. 59, 523–530 (2012).
Willis, F., Summers, J., Minutillo, C. & Hewitt, I. Indices of renal tubular function in perinatal asphyxia. Arch. Dis. Child. Fetal Neonatal Ed. 77, F57–F60 (1997).
Aggarwal, A., Kumar, P., Chowdhary, G., Majumdar, S. & Narang, A. Evaluation of renal functions in asphyxiated newborns. J. Trop. Pediatr. 51, 295–299 (2005).
Acknowledgements
We thank the NeuroNICU at Lucile Packard Children’s Hospital Stanford for assistance with archiving of NIRS data. A.F. is supported by National Institutes of Health (K23 HD079557).
Author information
Authors and Affiliations
Contributions
V.Y.C. substantially contributed to the conception and design of the study, acquisition of data, analysis and data interpretation, drafting the manuscript, critical revision of the manuscript, and final approval. S.-H.C. substantially contributed to the acquisition of data, data analysis and interpretation, critical revision of the manuscript, and final approval. A.F. substantially contributed to the conception and design of the study, acquisition of data, analysis and data interpretation, drafting the manuscript, critical revision of the manuscript, and final approval.
Corresponding author
Ethics declarations
Competing interests
A.F. is a scientific consultant for Takeda Pharmaceuticals. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chock, V.Y., Cho, SH. & Frymoyer, A. Aminophylline for renal protection in neonatal hypoxic–ischemic encephalopathy in the era of therapeutic hypothermia. Pediatr Res 89, 974–980 (2021). https://doi.org/10.1038/s41390-020-0999-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0999-y
This article is cited by
-
Renal oximetry for early acute kidney injury detection in neonates with hypoxic ischemic encephalopathy receiving therapeutic hypothermia
Pediatric Nephrology (2023)
-
Renal tissue oxygenation after caffeine administration in preterm neonates
Pediatric Research (2021)
-
Use of diuretics in the neonatal period
Pediatric Nephrology (2021)
-
A single dose of aminophylline administration during therapeutic hypothermia; does it make a difference in glomerular filtration rate?
European Journal of Pediatrics (2021)
-
Theophylline dosing and pharmacokinetics for renal protection in neonates with hypoxic–ischemic encephalopathy undergoing therapeutic hypothermia
Pediatric Research (2020)