Abstract
Background
Oxygen delivery during cardiopulmonary bypass (CPB) is closely related to postoperative acute kidney injury (AKI). The value of critical indexed oxygen delivery (DO2i) is a key indicator to reflect oxygen supply in cardiovascular surgery. However, the target DO2i value for neonates undergoing hypothermic CPB remains unclear.
Methods
One hundred and twenty-six consecutive newborns (≤28 days) undergoing arterial switch operations were retrospectively divided into two groups according to AKI occurrence. Baseline characteristics, intraoperative variables, and clinical outcomes were collected. Multivariate logistic regression analysis and receiver-operating characteristic curve were performed to investigate the association between DO2i and AKI.
Results
Neonates in the no-AKI group (n = 67) had significantly higher nadir bypass flow and DO2i during the hypothermic phase compared with the AKI group (n = 59). AKI group had remarkably higher incidences of hepatic dysfunction and peritoneal dialysis requirement compared with newborns without AKI. Mixed venous oxygen saturation (SvO2) was comparable between the two groups. Base excess (BE)(P = 0.011) value during the hypothermic phase of the AKI group was higher than the no-AKI group. Multivariate analysis showed that hypothermic DO2i was negatively associated with AKI. The cut-off value of hypothermic DO2i was 269 mL min−1 m−2.
Conclusions
The importance of hypothermic DO2i should be highlighted, even when SvO2 was satisfactory. A lower threshold of DO2i > 269 mL min−1 m−2 may help protect neonates from the risk of postoperative AKI.
Impact
-
The key message of our article is that the lower threshold of DO2i > 269 mL min−1 m−2 may help protect neonates from the risk of AKI after on-pump hypothermic cardiovascular surgery.
-
The critical DO2i value for neonates undergoing hypothermic CPB remains unclear, and our study may add new evidence for this matter based on the 6-year experience of our center.
-
In this study, the lowest critical value of DO2i in neonatal hypothermic CPB is determined for the first time, which provides a reference for intra-CPB management strategy to improve the postoperative outcomes of newborns.
Similar content being viewed by others
Introduction
Acute kidney injury (AKI) is one of the most common adverse events after pediatric cardiopulmonary bypass (CPB), and demands increased attention as it is closely related to postoperative mortality.1,2 Compared with older patients, neonates are much more vulnerable to CPB exposure and postoperative AKI.3,4 Neonatal CPB is associated with nonpulsatile blood flow, a systemic inflammatory state, and periods of low flow, which alter global oxygen delivery and may result in renal ischemia–reperfusion injury. Adequate systemic perfusion during CPB is clinically assumed when proper regional oxygen saturation (rSO2), mean arterial pressure (MAP), arterial lactate, base excess (BE), and mixed venous oxygen saturation (SvO2) levels are maintained. Nevertheless, even if these indexes are maintained within satisfactory levels, there still exists a high AKI incidence in neonatal patients.
As the key indicator of goal-directed perfusion (GDP), the indexed oxygen delivery (DO2i) combining CPB flow and hemoglobin (Hb) has been recently proposed as a supplement to intraoperative monitoring indexes in adults.5 Recent studies suggested that DO2i under 270 mL min−1 m−2 was correlated with increased incidence of postoperative AKI in adults undergoing hypothermic cardiac surgery,6 and for neonates undergoing normothermic CPB, the lower limit was 340 mL min−1 m−2.3 Besides, bypass flow rate of 2.4 L min−1 m−2 is typical for adults,7 and pediatric flow commonly ranges between 100 and 150 mL kg−1 min−1 (~1.6–2.4 L min−1 m−2) among different cardiac centers.8 However, due to the complexity of cardiovascular surgery and the particularity of the newborn population, there still lacks data indicating the critical values of DO2i and CPB flow for neonatal AKI after hypothermic CPB. Therefore, this study aims to explore the correlation between hypothermic CPB flow and DO2i with neonatal AKI, so as to offer step-forward evidence to optimize intraoperative management of neonatal cardiovascular surgery.
Materials and methods
Patients and study design
This study was approved by the Ethics Committee of Fuwai Hospital (approval number: 2014-600); informed consent from guardians was waived because of the retrospective nature of this study.
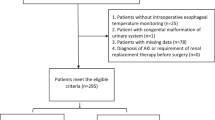
Medical records of all neonatal patients (≤28 days) undergoing one-stage on-pump arterial switch operations (ASOs) from November 1, 2012 to November 30, 2018 at Fuwai Hospital were retrospectively examined. Exclusion criteria were as follows: (1) age >28 days; (2) reoperation or secondary operation; (3) preoperative extracorporeal membrane oxygenation (ECMO) support; (4) emergency operation; (5) other existing aortic or pulmonary vascular malformations; (6) data missing.
Baseline information including demographics, preoperative echocardiographic examination, vasoactive inotropic score (VIScore), Risk Adjustment for Congenital Heart Surgery-1 (RACHS-1) classification,9 associated cardiovascular lesions, anemia (Hb < 145 g L−1), blood routine and biochemical examination, estimated glomerular filtration rate (eGFR) based on Schwartz’s formula {eGFR [mL/(min·1.73 m2)] = 0.45 × body length (cm)/[creatinine (μmol L−1)/88.4]}, and intraoperative variables covering CPB flow rates, CPB duration, ultrafiltration, body temperature, MAP, intraoperative medications, and arterial blood-gas analysis of each patient was collected. Noteworthily, nadir bypass flow rate during CPB hypothermic phase (≤30 °C) and rewarming phase (>30 °C) were collected separately.
DO2i was calculated from CPB flow rate, body surface area (BSA), and blood gas monitoring at two representative time points of CPB: when body temperature dropped to the lowest and CPB flow was stable during hypothermia period; 5 min after the opening of ascending aorta during rewarming period. The calculation formula is: DO2i (mL min−1 m−2) = pump flow × CaO2/BSA = pump flow (L min−1) × 1.36 × SaO2 (%) × Hb (g L−1)/BSA (m2).
Postoperative AKI was diagnosed according to the neonatal modified-Kidney Disease: Improving Global Outcomes (KDIGO) criteria.10
Anesthesia and monitoring
General anesthesia was induced. After endotracheal intubation for mechanical ventilation, central venous catheter, pulmonary artery catheter, and radial artery cannulation were placed under the guidance of ultrasound. Five-lead electrocardiogram (ECG), pulse oxygen saturation, intraoperative rectal temperature, bispectral index, and urine volume were routinely monitored throughout the surgical procedure.
Surgical and CPB management
The standard CPB was established with a roller pump (Stockert S5, Sorin, Germany) and an oxygenator (Sorin Kids D100, Sorin, Germany) following systemic heparinization and standard median sternotomy. CPB circuit was primed with 40 mL Plasmalyte A, 30 mL albumin, 80 mL packed red blood cells, and 10 mL sodium bicarbonate. A measure of 50–60 mL kg−1 cold histidine-tryptophan-ketoglutarate solution was continuously perfused for as body temperature cooled down to moderate hypothermia (27–30 °C). The surgeon cut open the right atrium before clamping the ascending aorta and suck the cardioplegia into the cell saver with an aspirator during perfusion to prevent the cardioplegia from flowing back into the circuit. The fixed surgeon team reconstructed the main arterial deformity during the hypothermia phase, after which the intracardiac concomitant lesions were repaired concurrently with rewarming. During bypass, α-stat management was conducted. The hematocrit (Hct) target was 0.24–0.27, and colloid osmotic pressure (COP) was maintained within 12–16 mm Hg during the procedure. Intra-CPB flow rate was adjusted to match the ideal value of SvO2 (≥70%), MAP (25–50 mm Hg), lactate (<3 mmol L−1), and BE [−3,3]. If MAP raises to >50 mm Hg, after excluding insufficient narcosis, we would use a sevoflurane inhalation device installed on the CPB system to reduce blood pressure slightly. Patients were weaned from CPB only when hemodynamic state and body temperature reached satisfactory levels as sinus rhythm returned to normal. After modified ultrafiltration, Hct was targeted at 0.35–0.40, and COP was maintained within 18–22 mm Hg. All newborns were transferred to the pediatric intensive care unit (PICU) after surgery and cared for by the fixed newborn critical care team.
Recovery outcomes and complications
Primary outcomes were: (1) in-hospital mortality; (2) postoperative AKI; (3) low cardiac output syndrome; (4) hepatic dysfunction defined as total bilirubin level >2.5 mg dL−1 or >2-fold increase of alanine aminotransferase or aspartate aminotransferase from baseline level;11 (5) respiratory infection diagnosed as the following ≥3 factors: cough, dyspnea, body temperature >38 °C, leukocyte >10 × 109/L or <4 × 109/L with or without nuclear left shift, and pulse >100 b.p.m.
Secondary outcomes included postoperative hospital stay, prolonged hospital stay (≥14 days), PICU length of stay, mechanical ventilation duration, delayed extubation (≥48 h), peritoneal dialysis, postoperative ECMO support, delayed sternal closure, and VIScore at PICU arrival.
Thirty-day follow-up echocardiography results of all patients were recorded.
Statistical analysis
Data analysis was performed using SPSS 25.0 software (SPSS Inc., Chicago, IL, USA) and figures were processed by GraphPad prism 8.0 (GraphPad Software Inc., San Diego, CA, USA). Categorical variables were reported as frequencies (percentage), and continuous variables were shown as mean ± standard deviation (SD) for normal distribution and median [interquartile range (IQR)] for abnormal distribution. Independent-sample Student’s t test and Mann–Whitney U test or Pearson χ2 test and Fisher’s exact test were used when appropriate for comparison between groups. Univariate and multivariate logistic regression analyses were used to evaluate the association between multi-perioperative variables and AKI during hypothermia. The receiver-operating characteristic (ROC) curve was used to examine the predictive value of significant indexes in logistic regression analysis for AKI, and the maximum Youden index was calculated to determine the cut-off value. P < 0.050 was considered statistically significant.
Results
Totally 126 cases were enrolled in this study and eight cases were excluded based on inclusion and exclusion criteria. Details were shown in Fig. 1.
Baseline characteristics
A total of 59 (46.83%) neonates were diagnosed with postoperative AKI. Cardiovascular surgeries of all newborns were moderate risk, ranking RACHS-1 grade 3 or 4. Baseline characteristics were comparable between the two groups except that the proportion of ventricular septal defect (VSD) was higher in the AKI group than in the no-AKI group (P = 0.020) (Table 1 and Supplementary Table 1).
Intraoperative variables
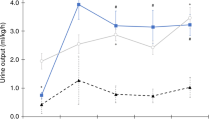
Nadir CPB flow rate during the hypothermic phase was significantly higher in the no-AKI group compared with the AKI group (123.09 ± 21.27 vs. 113.19 ± 21.87, P = 0.011). Hypothermic DO2i of the no-AKI group was statistically higher than the AKI group (226.53 ± 57.22 vs. 200.84 ± 45.30, P = 0.007). Full flow perfusion (>100 mL kg−1 min−1) was adopted in both groups during rewarming. Of note, as the flow rate increased, DO2i increased significantly from hypothermia to rewarming phase in both groups (P < 0.050) (Fig. 2) while SvO2 presented no statistical difference between the two groups. Besides, neonates suffering from AKI had a remarkably longer cooling time than the no-AKI group (P = 0.040). The comparisons of other intraoperative variables did not reach a significant level (Table 2). As Fig. 3 illustrated, hypothermic phase lactate (P = 0.064) and BE (P = 0.011) of the AKI group were higher than the no-AKI group. In addition, a discrepancy of lactate value between the two groups displayed a more apparent tendency as CPB progressed (P > 0.050). Perioperative arterial blood-gas analysis was shown in Supplementary Table 2.
Clinical outcomes
In-hospital mortality was comparable between the two groups. Neonates with AKI had a higher incidence of postoperative complications, including delayed extubation, peritoneal dialysis, and delayed sternal closure, although the difference was not statistically significant (Table 3). Besides, hepatic dysfunction occurred in a total of 95 (75.40%) neonates, with a significantly higher percentage in the AKI group than in the no-AKI group (P = 0.008). No difference was found for LVEF and LVED at discharge and 1 month of follow-up.
Moreover, we compared fluid overload index (Table 3) on the day of surgery and postoperative biochemical examination results of the two groups, and found that postoperative day 1 serum creatinine (SCr) and blood urea nitrogen of the AKI group was significantly higher than no-AKI group (P < 0.050) (Supplementary Table 1).
Logistic regression analysis
Logistic regression analysis was used to explore correlations between multivariables and AKI. After adjusting for VSD, cooling time, hepatic dysfunction and MAP, nadir hypothermic CPB flow rate (odds ratio (OR) 0.978, 95% confidence interval (CI) 0.960–0.996, P = 0.018), and DO2i during hypothermia (OR 0.991, 95% CI 0.983–0.998, P = 0.018) were independently negatively associated with neonatal AKI (Table 4). Univariate analysis of AKI was shown in Supplementary Table 3.
ROC analysis
ROC curves were used to detect the predictive value of nadir hypothermic CPB flow and DO2i during hypothermia for neonatal AKI. The area under the curve (AUC) of nadir hypothermic CPB flow was 0.620, and the cut-off value was 118.50 mL kg−1·min−1 (sensitivity 67.8%, specificity 55.2%) (P = 0.021). The critical value of DO2i during hypothermia to prevent AKI was 268.37 mL min−1 m−2 with an AUC of 0.631 (sensitivity 94.9%, specificity 32.8%) (Fig. 4).
Discussion
In this study, we investigated the correlation between nadir hypothermic bypass flow rate, DO2i, and postoperative AKI in neonates undergoing ASO surgeries. It was a relatively large neonatal case–control study concerning that so far there was no report on the minimum threshold of DO2i and nadir bypass flow rate during hypothermic CPB newborn population. In our results, newborns with AKI after hypothermic CPB may experience more adverse events, including hepatic dysfunction and dialysis requirement. The novel discovery was that maintaining a DO2i during hypothermia over the critical value of 269 mL min−1 m−2 may be warranted to prevent neonatal AKI.
The kidney is one of the most sensitive and vulnerable organs in cardiovascular surgeries.12 Renal medullary ischemia, mainly caused by low renal blood flow (RBF),13 has been identified as a major etiology of AKI.14 Newborns are susceptible to renal hypoperfusion. Compared with 20–25% of cardiac output received by adult kidneys, RBF in newborns accounts for <15% of CO.10 To evaluate the incidence of AKI in neonates, we employed the neonatal modified KDIGO criteria that incorporated key indicators in Risk, Injury, Failure, Loss, End-Stage (RIFLE) and Acute Kidney Injury Network (AKIN) criteria.15 The effectiveness of the KDIGO criteria has already been corroborated in a wide range of pediatric critical patients including those with congenital heart diseases.16 In our study, the incidence of AKI was 46.83% in the neonatal population, which was consistent with previous reports that AKI occurred in 37–67% of pediatric patients after cardiac surgery.4,17
The pathogenesis of postoperative AKI is closely associated with low bypass flow and low Hb during CPB. Both of them would lead to insufficient oxygen supply. Together with vasoconstriction during CPB, the insufficiency in oxygen supply may undermine glomerular filtration function, resulting in SCr increase and lactate accumulation, which were consistent with our results. As Hb was managed >8.0 g dL−1 in this study, a higher flow may protect newborns against AKI by ameliorating oxygen delivery and clearing local metabolites.
Surgical repair and revascularization were mainly completed during hypothermia. Therefore, nadir CPB flow rate was creatively collected in two stages in this research: hypothermic phase and rewarming phase. According to multivariate analysis, higher nadir CPB flow rate and DO2i during the hypothermic phase were independent protective factors for neonatal AKI. Hypothermic bypass flow had exerted a greater influence than rewarming flow to patients. Hence, greater attention should be paid to CPB flow during hypothermia in neonatal surgeries in order to facilitate oxygen supply. Based on ROC analysis, with an Hct > 0.24, a nadir hypothermic flow rate >119 mL kg−1 min−1 may be appropriate for newborns.
Generally, RBF was comparatively abundant under higher CPB flow and dropped as CPB flow reduced. However, the hemodynamics of renal perfusion varied at different temperatures. RBF was usually sufficient and stable at mild hypothermia,18 while during the moderate hypothermic phase (28 °C) as in our study, RBF mainly relied on CPB flow19 partly due to hypoxic renal vasoconstriction. Renal oxygen delivery was primarily determined by RBF and Hb. Hemodilution during CPB ineluctably decreases oxygen delivery; however, intraoperative oxygen consumption is usually maintained at the preoperative level,20 thus renal oxygenation was mainly affected by CPB flow at that stage.
To date, there is no direct method to monitor renal oxygen pressure in pediatric cardiac surgeries.21 That, highlighting the importance of DO2i, which reflects the systemic oxygen supply. We calculated DO2i at two CPB phases: hypothermia and rewarming phase. DO2i of the no-AKI group was higher than that of the AKI group during hypothermia, and DO2i of both groups during rewarming was higher than during hypothermia. The recent adult study has shown that the probability of AKI increased for an average of 7% as DO2i decreased for every 10 mL min−1 m−2.6 Roughly speaking, our data suggested that a hypothermic DO2i > 269 mL min−1 m−2 may be beneficial to newborns, which was similar to the traditionally recognized lower limit of DO2i for adults in GDP with 260–272 mL min−1 m−2.6,22 The latest single-center observational research identified 353 mL min−1 m−2 as the nadir critical value for infants (1 month ≤ age ≤3 years) against AKI.23 We noticed that the intraoperative bypass flow was controlled at 2.8–3.2 L min−1 m−2 in their protocol, which was a value higher than most centers, reflecting an aggressive CPB management strategy. However, we are concerned about the effects of that high flow rate and potential hemolysis on renal function.
In our study, SvO2 during CPB was maintained at >75%. Svenmarker et al.24 demonstrated that SvO2 >75% can reduce the risk of AKI after CPB for adults. Whereas we did not find any difference in renal oxygenation reflected by SvO2 between the two groups. Therefore, SvO2 may be an unreliable indicator of the optimal perfusion and should be used in combination with DO2i for neonates.
Serum lactate concentration may reflect anaerobic metabolism and thus poor end-organ perfusion. As Abraham et al. reported, CPB flow <100 mL kg−1 min−1 may increase the risk of lactate concentration >3 mmol L−1 by >7-folds.25 In this study, lactate accumulated while BE values decreased, and gaps between groups were gradually more evident as CPB progressed, indirectly suggesting an imbalance in renal oxygen supply-and-demand. The possible mechanism was that potential RBF insufficiency arose from low CPB flow rendered microcirculatory perfusion deficiency and disorders of tissue oxygenation, which damaged mitochondria of tubular epithelial cells, thus resulting in adaptative anaerobic glycolysis. In the process of AKI, quantities of lactate produced by renal tubular cells through glycolysis were absorbed by interstitial fibroblasts. In vitro study indicated that activated fibroblasts produced collagen,26 which aggravated the formation of renal interstitial fibrosis. Therefore, low CPB flow may intensify the development of AKI and promote its transition to chronic kidney disease.
The effect of intraoperative arterial pressure on AKI after CPB remains inconclusive. Many scholars failed to find any correlation between MAP and renal injury.27,28 Considering the impact of hemodilution and physiological hypotension, intraoperative MAP of newborns was commonly maintained within 25–50 mm Hg in our center. Pediatric renal perfusion mainly depends on CPB flow, whereas adult patients seem to rely more on MAP, although it may be affected by many intraoperative factors. In line with that, our study showed that flow rate was correlated with AKI, even after adjusting for MAP.
About 30% of patients with complete transposition of great arteries are complicated with VSD. In this research, the AKI group had a higher incidence of VSD than the no-AKI group. Although intracardiac shunt caused by VSD may alleviate the symptoms of hypoxia to some extent, the associated decrease of systemic blood flow undermines renal oxygen supply, which would increase the sensitivity of kidneys to oxygen deficiency in hypothermic CPB, thus promoting the development of AKI.
Analysis of the data in previous studies led to the conclusion that magnitude and incidence of SCr elevation were significantly correlated with clinical outcomes, including mortality and multiorgan changes.29 However, postoperative adverse events concerning mortality, heart, lungs, and secondary complications presented no statistical difference between the two groups in our study. On the one hand, the reason may be adequate perioperative cardiac surgery and care techniques and the application of antibiotics. On the other hand, there was no difference in postoperative hospital stay and PICU stay between the two groups, which may be related to the relatively conservative postoperative nursing strategy of our center. Due to the immature development of the newborn liver, diagnosis of hepatic dysfunction that is merely based on laboratory examination may lead to misdiagnosis, and confirmation by ultrasound was needed.
There were certain limitations in the present study. Firstly, the single-center and retrospective nature had limited the intensity of evidence, even though the number of newborns was relatively large. Secondly, based on our center’s CPB protocol, the lack of multiple blood gas monitoring in the same CPB phase (hypothermia or rewarming) was a limitation of this study as a retrospective study. Thirdly, in our center, NIRS had only been routinely used to monitor cerebral or renal SO2 in pediatric cardiac surgeries since 2018. Data of rSO2 in this population was incomplete and therefore excluded from this study. Lastly, the subjects of this study were the neonatal subgroup of ASO, which means corroboration of our findings needs to be verified in a larger population or well-designed multicenter randomized controlled trials containing more surgical procedures before further promotion.
Conclusion
Bypass flow rate and DO2i during CPB hypothermia should be highly valued. Application of hypothermic DO2i >269 mL min−1 m−2 during neonatal CPB may exert protective effects against postoperative AKI. The lower threshold of CPB flow should be higher than the critical value of 119 mL kg−1 min−1 if intra-CPB Hct was targeted >0.24. If possible, we recommend the combined use of SvO2 and DO2i in neonatal CPB practice.
References
Caputo, M. et al. Normothermic versus hypothermic cardiopulmonary bypass in low-risk paediatric heart surgery: a randomised controlled trial. Heart 105, 455–464 (2019).
Ueno, K. et al. Kidney Disease: Improving Global Outcomes in neonates with acute kidney injury after cardiac surgery. Clin. Exp. Nephrol. 24, 167–173 (2020).
Bojan, M. et al. Lower limit of adequate oxygen delivery for the maintenance of aerobic metabolism during cardiopulmonary bypass in neonates. Br. J. Anaesth. 124 (2020).
Piggott, K. D. et al. Inadequate preoperative nutrition might be associated with acute kidney injury and greater illness severity postoperatively. J. Thorac. Cardiovasc. Surg. 155, 2104–2109 (2018).
Magruder, J. T. et al. Correlating oxygen delivery on cardiopulmonary bypass with Society of Thoracic Surgeons outcomes following cardiac surgery. J. Thorac. Cardiovasc. Surg.(2020) https://doi.org/10.1016/j.jtcvs.2020.12.008.
Newland, R. F., Baker, R. A., Woodman, R. J., Barnes, M. B. & Willcox, T. W. Australian and New Zealand Collaborative Perfusion Registry. Predictive capacity of oxygen delivery during cardiopulmonary bypass on acute kidney injury. Ann. Thorac. Surg. 108, 1807–1814 (2019).
Zhu, M. Z. L. et al. Urinary hypoxia: an intraoperative marker of risk of cardiac surgery-associated acute kidney injury. Nephrol. Dial. Transpl. 33, 2191–2201 (2018).
Joffe, R. et al. Cardiac surgery-associated kidney injury in children and renal oximetry. Pediatr. Crit. Care Med. 19, 839–845 (2018).
Thiagarajan, R. R. & Laussen, P. C. Risk Adjustment for Congenital Heart Surgery -1 (RACHS-1) for evaluation of mortality in children undergoing cardiac surgery. Pediatric and Congenital Cardiac Care. 1, 327–336 (2015).
Selewski, D. T. et al. Neonatal acute kidney injury. Pediatrics 136, e463–e473 (2015).
Tong, Y. et al. Perioperative outcomes of using different temperature management strategies on pediatric patients undergoing aortic arch surgery: a single-center, 8-year study. Front. Pediatr. 6, 356 (2018).
Mazzone, A. L., Bakerand, R. A. & Gleadle, J. M. Mending a broken heart but breaking the kidney. Nephrology 21, 812–820 (2016).
Evans, R. G. et al. Haemodynamic influences on kidney oxygenation: clinical implications of integrative physiology. Clin. Exp. Pharm. Physiol. 40, 106–122 (2013).
Lankadeva, Y. R. et al. Strategies that improve renal medullary oxygenation during experimental cardiopulmonary bypass may mitigate postoperative acute kidney injury. Kidney Int. 95, 1338–1346 (2019).
Thomas, M. E. et al. The definition of acute kidney injury and its use in practice. Kidney Int. 87, 62–73 (2015).
Selewski, D. T. et al. Validation of the KDIGO acute kidney injury criteria in a pediatric critical care population. Intens. Care Med. 40, 1481–1488 (2014).
Wang, C. et al. Epidemiology of acute kidney injury among paediatric patients after repair of anomalous origin of the left coronary artery from the pulmonary artery. Eur. J. Cardiothorac. Surg. 56, 883–890 (2019).
Lannemyr, L. et al. Effects of cardiopulmonary bypass on renal perfusion, filtration, and oxygenation in patients undergoing cardiac surgery. Anesthesiology 126, 205–213 (2017).
Andersson, L. G. et al. Renal function during cardiopulmonary bypass: influence of pump flow and systemic blood pressure. Eur. J. Cardiothorac. Surg. 8, 597–602 (1994).
Redfors, B., Bragadottir, G., Sellgren, J., Swärd, K. & Ricksten, S. E. Acute renal failure is NOT an “acute renal success”–a clinical study on the renal oxygen supply/demand relationship in acute kidney injury. Crit. Care Med. 38, 1695–1701 (2010).
Evans, R. G. et al. Renal haemodynamics and oxygenation during and after cardiac surgery and cardiopulmonary bypass. Acta Physiol. 222, e12995 (2018).
Mukaida, H. et al. Time-dose response of oxygen delivery during cardiopulmonary bypass predicts acute kidney injury. J. Thorac. Cardiovasc. Surg. 158, 492–499 (2019).
Zhang, Y., Wang, B., Zhou, X., Guo, L. & Zhou, R. Nadir oxygen delivery during pediatric bypass as a predictor of acute kidney injury. Ann. Thorac. Surg. (2021) https://doi.org/10.1016/j.athoracsur.2021.01.026.
Svenmarker, S., Hannuksela, M. & Haney, M. A retrospective analysis of the mixed venous oxygen saturation as the target for systemic blood flow control during cardiopulmonary bypass. Perfusion 33, 453–462 (2018).
Abraham, B. P. et al. Cardiopulmonary bypass flow rate: a risk factor for hyperlactatemia after surgical repair of secundum atrial septal defect in children. J. Thorac. Cardiovasc. Surg. 139, 170–173 (2010).
Wagner, S., Hussain, M. Z., Hunt, T. K., Bacic, B. & Becker, H. D. Stimulation of fibroblast proliferation by lactate-mediated oxidants. Wound Rep. Regen. 12, 368–373 (2004).
Lannemyr, L., Bragadottir, G., Hjärpe, A., Redfors, B. & Ricksten, S. E. Impact of cardiopulmonary bypass flow on renal oxygenation in patients undergoing cardiac operations. Ann. Thorac. Surg. 107, 505–511 (2019). p.
Haase, M. et al. Effect of mean arterial pressure, haemoglobin and blood transfusion during cardiopulmonary bypass on post-operative acute kidney injury. Nephrol. Dial. Transplant. 27, 153–160 (2012).
Waikar, S. S. & Bonventre, J. V. Creatinine kinetics and the definition of acute kidney injury. J. Am. Soc. Nephrol. 20, 672–679 (2009).
Acknowledgements
We appreciate Mr. Ricardo Annon and Dr. Yue Yuan for their great assistance in language polishing. This work was financially supported by the National Natural Science Foundation of China Grant Awards (81670375). The scientific abstract of this paper was accepted for oral presentation on 3rd October at the American Society of Anesthesiologists (ASA) 2020 Annual Meeting (Online).
Author information
Authors and Affiliations
Contributions
J.L. conceived the primary research question. P.Z. and J.L. designed the study. P.Z. drafted the manuscript. Y.T. collected data and revised the manuscript. P.Z., Y.T., J.L., S.G., L.B., Y.J., Y.L., Z.F., and J.Z. provided critical revision to the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
This study was approved by the Ethics Committee of Fuwai Hospital (approval number: 2014-600); informed consent from guardians was waived because of the retrospective nature of this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Zhang, P., Tong, Y., Liu, J. et al. The lower threshold of hypothermic oxygen delivery to prevent neonatal acute kidney injury. Pediatr Res 91, 1741–1747 (2022). https://doi.org/10.1038/s41390-021-01654-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01654-9
This article is cited by
-
Renal Outcomes in Neonates and Infants with Transposition Physiology Undergoing Arterial Switch Procedure
Pediatric Cardiology (2022)