Abstract
Background
The prevalence of autism spectrum disorders (ASD) is 5-fold higher in preterm (PT) infants born ≤28 weeks gestational age (GA) as compared to the general population. The relationship between placental pathologic lesions and ASD in PT infants has not been studied.
Objectives
The objective of this study was to determine the association of placental pathology with the occurrence of ASD in PT infants born ≤28 weeks GA.
Study design
A matched case–control study to identify confirmed ASD cases (n = 16) and matched controls (n = 48) born at Parkland Hospital between January 2012 and December 2015. Patients were matched using known variables associated with increased risk of ASD in PT infants. Placental histology from all births was reviewed.
Results
Children with ASD had 2-fold greater incidence of multiple placental pathologic lesions vs. matched controls [11/16 (69%) vs.16/48 (33%), respectively; P = 0.01]. In contrast, single placental pathologic lesions were not associated with ASD [5/16 (31%) vs. 21/48 (43%), respectively; P = 0.1].
Conclusions
In this study, we have demonstrated an association between the increasing complexity of histologic placental lesions and the later risk for ASD in infants born ≤28 weeks GA. Thus, placental pathology findings may be valuable in further understanding the prenatal pathologic processes underlying ASD in PT infants.
Impact
-
PT infants with ASD have a 2-fold greater incidence of multiple placental pathologies.
-
This is the first study to report an association between the complexity of histologic placental lesions and later risk of ASD in infant born extremely PT (i.e., ≤28 weeks GA).
-
This study reiterates the importance of examining placental pathologic lesions, since placental evidence of antenatal insults correlates with postnatal morbidities and mortality in PT infants.
Similar content being viewed by others
Introduction
Autistic spectrum disorder (ASD) is a neurodevelopmental disorder in the category of pervasive developmental disorders and is characterized by severe and pervasive impairment in reciprocal socialization, qualitative impairment in communication, and repetitive or unusual behavior.1 Children born significantly preterm (≤28 weeks gestational age; GA) are at substantially increased risk of ASD.2,3,4,5,6 The prevalence of ASD in children born extremely preterm is 7.1% versus 1.5% in the general US population.6,7 ASD risk decreases with increasing GA,6,8 15.0% for 23–24 weeks, 6.5% for 25–26 weeks, to 3.4% for 27 weeks GA.6 The association between a child’s ASD risk and lower GA is independent of intelligence quotient and global neurodevelopmental delays.6 Low GA is a marker of the immaturity and vulnerability of the central nervous system as well as other physiological systems that function to protect the developing brain and, when perturbed, put the developing fetus at risk.9,10,11 Thus, variables that promote preterm birth might also be related to the development of ASD. For example, maternal and fetal inflammation associated with processes leading to extremely preterm birth12,13,14 appear to be antecedents of ASD.15,16
Although the placenta is of fetal origin and composed solely of fetal cells, its blood supply is derived from the maternal uterine and fetal umbilical arteries, the former serving as the source of placental and fetal oxygen and nutrient delivery.17 Both the placenta and fetus grow in the same intrauterine environment during pregnancy, hence placental evidence of antenatal insults correlates with morbidities and mortality in preterm infants.18 Changes in placental histology may be associated with altered placental function, and thus modification in fetal outcomes.19,20 As such, the placenta is uniquely positioned to directly influence fetal programming. However, the placenta remains an underutilized source of information about exposures during pregnancy.
Interestingly, many of the known risk factors for ASD are associated with evidence of placental inflammation and other placental pathologic lesions. For example, preterm birth, maternal obesity, elevated cytokines in amniotic fluid, pregestational diabetes, and maternal infection during pregnancy are all risk factors for the occurrence of ASD that are also associated with the presence of placental inflammation or other placental pathologic lesions.21,22,23,24,25,26,27
Recently, Straughen et al.28 reported that histologic evidence of placental inflammation and maternal vascular malperfusion pathology are associated with increased risk of ASD in children born at term or near term. However, they did not correct for multiple placental pathologic lesions (the presence of ≥2 pathologic lesions) and did not study extremely preterm infants, that is, born ≤28 weeks GA. Raghavan et al.29 reported increased incidence of ASD in preterm infants (<37 weeks GA) with placental pathology. However, they also did not examine the occurrence of multiple placental pathologic lesions and did not specifically study the extremely preterm infants. Thus, the association of placental pathologic lesions with definitive diagnosis of ASD in preterm children born ≤28 weeks GA is not known. We hypothesize that multiple placental pathologies are associated with increased incidence of ASD in preterm infants born ≤28 weeks GA. Accordingly, the objectives of this study were to determine the association of placental pathology, including multiple placental pathologic lesions with ASD in preterm infants born ≤28 weeks GA by using a uniform classification of placental histopathology.
Methods
Study design
A matched case–control study was conducted, examining children with a diagnosis of ASD who were born ≤28 weeks GA. All the children were born between January 2012 and January 2015 at Parkland Hospital and Health Systems, Dallas, Texas. The study was approved by the Institutional Review Board at the University of Texas Southwestern Medical Center and Parkland Health and Hospital System.
ASD assessment
Children born ≤28 weeks GA at Parkland Hospital routinely undergo systematic standardized neurologic assessments and the Bayley Scales of Infant and Toddler Development, Third edition (Bayley III) at 22–26 months corrected age.30,31 They are also screened for ASD using M-CHAT (The Modified Checklist for Autism in Toddlers) screening tool at 24 months of age.32 M-CHAT is a widely used simple parental screen that consists of 23 yes/no items.32 Optimal age for administration is 18–24 months. There are six critical questions and 17 non-critical questions. Children are considered to have screened positive if they fail ≥2 of the six critical items. These children were assessed at ~4 years of age by a neuropsychologist using either the Autism Diagnostic Observation Schedule, Second edition (ADOS-2)33 and/or Childhood Autism Rating Scale, Second Edition (CARS-2)34 as part of a more comprehensive assessment for a definitive diagnosis of ASD. These two measures were chosen, in particular, as they are among the most commonly used objective measures of autism. The ADOS-2 is considered a gold standard measure in the assessment of ASDs and consists of a standard set of interactions and activities that sample the child’s social interaction, communication, and behaviors. One of five modules is administered depending on the age and expressive language skills of the person being evaluated and provides cut-off scores for autism and ASD classifications. The CARS-2 is a 15 item behavioral scale developed to identify children with autism and has two forms: one for individuals assessed to have average or above cognitive skills and deemed verbally fluent, and one for individuals with expressive communication difficulties and/or below average cognitive skills. The CARS-2 rates each of the behaviors according to the frequency, intensity, duration, and abnormality with higher scores are indicative of severe symptoms of autism.
Electronic health records of all children born ≤28 weeks GA between January 2012 and January 2015 at Parkland Hospital were reviewed. We searched for the terms autism or Autistic Disorder or Asperger Syndrome or Autistic Disorder or Pervasive/or pervasive developmental disorder in their electronic medical records. The charts of children with any of these presumed diagnosis were reviewed by a psychologist (S.P.W.) to determine whether or not an ASD diagnosis was definitive based on available data in record (i.e., definitively diagnosed by a medical or psychological professional with assessment data to support).
Controls were matched (1:3) for GA, sex, gestational diabetes, and maternal age, as there is evidence that these variables are associated with increased risk of ASD in children born preterm.35 Differences in maternal demographic and clinical variables by ASD status were assessed using conditional logistic regression in order to account for the matching inherent in the study design.
Placental pathology
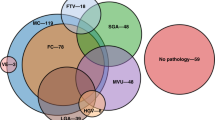
Placentas from all preterm births at Parkland Hospital are routinely sent for gross and histologic examination by a placental pathologist according to a standing protocol previously described.36 In brief, initial gross examination of the umbilical cord, membranes, and placental disc is first performed. Placental weight is obtained after removal of the umbilical cord, fetal membranes, and non-adherent blood clots.37 The placental disk is sectioned at 1–2 cm intervals and grossly examined for intraparenchymal lesions. Representative sections of the umbilical cord, fetal membranes, placental parenchyma, and any abnormalities seen on gross exam are obtained and submitted for standard histological examination. The pathologists at our institution use the Redline classification for major placental findings.38 A priori definition of a “significant placental pathology” was determined by an independent pathologist blinded to the clinical outcomes. This definition is standardized at our institution and excludes the minor placental pathology in each major category of placental pathologies that show little or no clinical consequence and are present in most of the placental histological examinations of normal term neonates. This definition of “significant placental pathology” is based on modified Redline classification, published by our group in 2015.39 “Significant placental pathology” includes the following placental lesions: (1) acute histologic chorioamnionitis with/without fetal inflammatory response: presence of maternal inflammatory responses (acute subchorionitis or acute chorioamnionitis) with or without fetal inflammatory responses (vasculitis in the umbilical vessels and/or chorionic plate vessels) was recorded as chorioamnionitis. The presence or absence of fetal inflammatory response was separately recorded.40 (2) High-grade villitis (HGV): patchy/diffuse chronic villitis with or without obliterative fetal vasculopathy; chronic villitis was defined as the presence of chronic inflammation of chorionic villi.41 High-grade lesions (HGV) included inflammatory foci involving >10 villi/focus, either limited in distribution (patchy) or more extensive (diffuse).42 (3) Maternal vascular underperfusion (MVU); MVU was defined by distal villous hypoplasia, severe maternal decidual vasculopathy (fibrinoid necrosis and/or acute atheromatous changes), and/or infarcts involving >20% of the placental volume.39,43 (4) Fetal thrombotic vasculopathy (FTV); the two variables of large vessel thrombi and villous damage from reduced fetal perfusion (avascular villi or villous stromal vascular karyorrhexis) were grouped under the heading of FTV.44 (5) Patchy/diffuse villous edema (VE); the morphologic variable of VE (patchy/diffuse VE) was added to the classification, as it might represent fetal hypoxia, or feto-maternal hemorrhage.45,46 (6) Small for GA (SGA)/large for GA (LGA) placentas;47 trimmed placental weight <10th percentile was defined as SGA placenta and >90th percentile was defined as LGA placenta.47 The presence of any one of the six lesions described was defined as significant placental pathology without overlap. The presence of >1 placental lesion noted above was classified as overlapping placental pathology. We previously reported 100% concordance between the routine placental pathology report and independent review of placenta with blinded pediatric pathologist (30). All placental pathology reports were reviewed by a pediatric pathologist and neonatologist (Sarah Johnson-Welch and I.N.M.), while blinded to clinical history and the outcomes. All study patients were divided into three groups based on placental pathology: (1) no placental pathology; (2) significant placental pathology without overlapping lesions; and (3) overlapping placental pathology (presence of >1 significant placental pathologic lesion).
Data collection
Baseline maternal and infant characteristics, morbidities, and mortality were compared. In addition to examination of the electronic health record, data were obtained from the Parkland NICU Database that is prospectively collected from all neonates admitted to the Parkland Hospital NICU.
Statistical analysis
SPSS version 19 (IBM) was used to perform statistical analyses. Descriptive statistics were calculated to compare neonates between the two groups as described above. Categorical variables were analyzed by χ2 test. Continuous variables were analyzed by Student’s t test. A two-sided 0.05 level of significance was used for all analyses. Differences in maternal demographic and clinical variables by ASD status were assessed using conditional logistic regression in order to account for the matching inherent in the study design. In the logistic regression for multiple placental pathologic lesions, we controlled for matching variables between cases and controls, that is, GA, sex, gestational diabetes and maternal age. Odds ratios (ORs) and 95% confidence intervals (CIs) were also calculated using conditional logistic regression. When models did not converge, for example, due to cells with zero observations, we used logistic regression, included the matching variables as covariates, and used smoothing. In such instances where there were cells with zero observations, data were smoothed by adding a single observation for zero cells with covariate values set to the mean for that outcome category for that cell.
Results
Study population
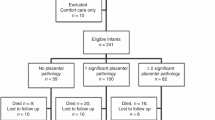
Between January 2012 and January 2015, 248 neonates were born at 23–28 weeks GA. After excluding neonates who received comfort care, 238 were included. Out of them, 218 completed the follow-up and underwent systematic standardized neurologic assessments, the Bayley III and M-CHAT screening at 22–26 months corrected age. Thirty-one (14%) were observed to have ASD/Autism recorded in their electronic health records. Fifteen of 31 children had either a M-CHAT screen positive or suspected ASD/Autism in their Problem List. Sixteen had a formal diagnosis of ASD at ~4 years of age by a neuropsychologist using CARS-2 ST and/or ADOS-2 (Fig. 1), resulting in a prevalence of ASD in preterm infants born ≤28 weeks GA of 7.3%. The majority of cases were male (69%). Cases and matched controls did not significantly differ in any maternal and/or neonatal characteristics (Table 1).
Association of placental pathology with ASD
Acute histologic chorioamnionitis was the most prevalent placental pathologic lesions in ASD cases and matched controls [10/16 (63%) and 25/48 (52%), respectively; P = 0.47]. The ASD group had an increased occurrence of LGA placentas vs. control group [5/16 (31%) vs. 2/48 (4%), respectively, P < 0.01]; otherwise, no other single placental pathologic lesions was associated with ASD vs. matched controls [5/16 (31%) vs. 21/48 (43%), P = 0.1] (Table 2). In contrast, children with ASD had 2-fold greater occurrence of multiple placental pathologic lesions vs. matched controls [11/16 (69%) vs.16/48 (33%), respectively; P = 0.01]. Logistic regression analysis revealed that the presence of multiple pathologic lesions was significantly associated with an increased risk for later diagnosis of ASD [OR = 6.5 (CI 1.6, 27.1). Among the multiple placental lesions, a combination of LGA placentas + acute histologic chorioamnionitis with/without fetal vasculitis was associated with the occurrence of ASD vs. controls [4/16 (25%) vs. 0/48, respectively, P < 0.01] (Table 3).
Other outcomes
The incidence of other neonatal morbidities and neurodevelopmental impairment (NDI) did not differ between ASD cases and matched controls [grade IV IVH 1(6%) vs. 5 (10%), P = 0.6; necrotizing enterocolitis 1(6%) vs. 3(6%), P = 1; bronchopulmonary dysplasia 9 (56%) vs. 27 (56%), P = 1; NDI 9(60%) vs. 19 (53%), P = 0.6, respectively] (Table 4).
Discussion
In this study, we examined the relationship between placental pathology, including multiple placental pathologic lesions, and ASD in preterm infants ≤28 weeks GA. The major finding of the study is that children born extremely preterm with a definitive diagnosis of ASD had a 2-fold greater incidence of multiple placental pathologic lesions. Specifically, the combination of large for GA placentas (placental overgrowth) and histologic chorioamnionitis with fetal vasculitis was associated with the later diagnosis of ASD in this unique patient population. Placentas from ASD cases were large for GA; otherwise, no other specific placental pathologic lesion was associated with increased risk for ASD. We also observed that M-CHAT screening for ASD did not have a good positive predictive value for definitive diagnosis of ASD in children born extremely preterm at ~4 years of age.
When considered in the context of existing literature, our findings are consistent with Straughen et al.,28 who noted that histologic evidence of both acute and chronic placental inflammation, as well as maternal vascular malperfusion pathology, were associated with increased risk of ASD. However, Straughen et al. did not study the effects of placental pathology in children born preterm, which by itself is an important risk factor for the occurrence of ASD.6 In another study, using data from the Avon Longitudinal Study of Parents and Children, a population-based birth cohort, Salafia et al.,48 reported that compared to placentas of control infants, placentas of children with ASD had a statistically significant 40% reduction in chorionic surface vascular branch points, with 2 fewer branch generations and reduced extension of the surface vessels to the chorionic plate edge. This study also was restricted to only infants born term and near term. To our knowledge, only Raghavan et al.29 examined the association of placental pathology with the incidence of ASD in preterm infants; however, they included a broader range of preterm births (<37 weeks GA) and did not report on infants born extremely preterm, in whom the prevalence of ASD is four times higher than in the general population.
The underlying mechanism for greater likelihood of ASD among extremely preterm babies with placental pathologies is complex and poorly understood. It is possible that the presence of inflammatory placental pathologic lesions are associated with the activation of a fetal inflammatory response and release of proinflammatory cytokines that could directly impact the immature brain and lead to neuroinflammation and increase susceptibility to neurodevelopmental disorders, including ASD. Evidence suggests that histologic chorioamnionitis is associated with the presence of white matter brain lesions.49 Independent studies have shown that white matter alterations are seen in ASD, suggesting a underlying neural mechanism for this disorder.50 Alternatively, chronic placental pathologic lesions that manifest early in pregnancy may alter placental angiogenesis and have downstream effects on fetal angiogenesis and neurogenesis.51 Moreover, chronic exposure to low-level inflammation,52 hyperoxia,53 and hypoxia54 during development is associated with changes in genes associated with synapse structure and function.55 Furthermore, behaviors such as reduced social play behavior and increased repetitive grooming are impaired in a recently described rat model that combined the injuries of inflammation and hypoxia.56
Interestingly, placentas from ASD cases in our study were large for GA (placental overgrowth) as compared to controls. Studies of the effects of famine on maternal, fetal, and placental weight have long ago demonstrated that the weights of placentas and babies decline when maternal nutrition is lacking.57 However, the timing of exposure to famine seems important in placental growth. In mothers who experienced famine around the time of conception or in the first trimester of pregnancy, placental weight was greater than expected for the fetal weight,58 thus following a placental overgrowth phenotype with decreased placental efficiency. Additional studies that demonstrated compensatory placental growth in the suboptimal host include women with anemia.59 In a guinea pig model of maternal hypoxia, investigators demonstrated large placentas, due to increased trophoblast cell proliferation, but decreased migration and invasion of maternal vessels.60 This model showed a higher placental to birth weight ratio indicative of placental overgrowth and placental inefficiency. Placental overgrowth might act as a source of vascular steal, forcing the fetal heart to perfuse the larger placenta at the expense of fetal somatic and brain perfusion. Further studies are needed to more fully characterize the placental overgrowth phenotype and its effect on fetal development.
An additional finding of our study was that only ~50% of children born ≤28 weeks GA with positive M-CHAT screening for ASD at 24 months of age a had definitive diagnosis of ASD at ~4 years of age. This is consistent with recent reports.61,62 In a recent study, Kim et al.61 reported that in preterm infants M-CHAT had a sensitivity of 52%, a specificity of 84%, and a positive predictive value of 20%. In other words, among extremely preterm children with ASD, almost one-half were not correctly identified by the M-CHAT at 2 years of age.61 As reported by others, the M-CHAT should be interpreted cautiously when used to screen extremely preterm infants, suggesting that the developmental impairments frequently observed in this patient population may explain poor prediction by M-CHAT screening tool. It also highlights the importance of thoughtful evaluation of M-CHAT results—including, perhaps, a review of the particular items acknowledged by the caregiver. Perhaps, the M-CHAT needs to be modified for use in preterm infants in order to improve the specificity of this assessment tool. Thus, M-CHAT results should be interpreted with caution in screening preterm infants. Given the neurodevelopmental complexity of these children, a more comprehensive assessment for ASD would also be recommended.
The strengths of our study include a relative homogeneity of the study subjects delivered and cared for at a single center, high rate of follow-up (~90%) with systematic assessment of long-term outcomes, and availability of placental histopathology in all preterm infants delivered in our institution. We also assessed a large variety of placental lesions, defined their significance a priori, used a uniform method for classification that has been validated, and assessed multiple lesions. Our study has some limitations, including its retrospective nature and relatively small sample size. Even though logistic regression was used to account for known variables, it is possible that unknown confounders were not accounted for. It is highly likely that multiple placental lesions indicate an interaction between vascular and inflammatory lesions; but they may also indicate severity of the vascular or inflammatory insult. Future studies should explore role of such lesions and the interaction in pathogenesis of neonatal morbidities.
In summary, using a standardized placental pathology classification, this matched case–control study showed that the presence of multiple placental pathologic lesions increased the odds of ASD occurring in extremely preterm infants. Further, our findings support the emerging theory that prenatal risk factors may contribute to the increased risk of ASD in extremely preterm infants. Our findings suggest that placental pathology findings may be valuable in shedding light on prenatal pathologic processes underlying ASD. These observations should stimulate the design of future clinical and mechanistic studies that could provide further insight into our understanding of ASD in the preterm infants. Importantly, our findings suggest that M-CHAT results in children born extremely preterm should be interpreted with caution, and a more comprehensive assessment for ASD would be recommended as a future target.
References
Levy, S. E., Mandell, D. S. & Schultz, R. T. Autism. Lancet 374, 1627–1638 (2009).
Hack, M. et al. Behavioral outcomes of extremely low birth weight children at age 8 years. J. Dev. Behav. Pediatr. 30, 122–130 (2009).
Johnson, S. et al. Autism spectrum disorders in extremely preterm children. J. Pediatr. 156, 525–31. e2 (2010).
Pinto-Martin, J. A. et al. Prevalence of autism spectrum disorder in adolescents born weighing <2000 grams. Pediatrics 128, 883–891 (2011).
Treyvaud, K. et al. Psychiatric outcomes at age seven for very preterm children: rates and predictors. J. Child Psychol. Psychiatry 54, 772–779 (2013).
Joseph, R. M. et al. Prevalence and associated features of autism spectrum disorder in extremely low gestational age newborns at age 10 years. Autism Res. 10, 224–232 (2017).
Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators; Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill. Summ. 63, 1–21 (2014).
Kuzniewicz, M. W. et al. Prevalence and neonatal factors associated with autism spectrum disorders in preterm infants. J. Pediatr. 164, 20–25 (2014).
Dammann, O. & Leviton, A. Brain damage in preterm newborns: might enhancement of developmentally regulated endogenous protection open a door for prevention? Pediatrics 104, 541–550 (1999).
Leviton, A. et al. The wealth of information conveyed by gestational age. J. Pediatr. 146, 123–127 (2005).
Sanders, E. J. & Harvey, S. Peptide hormones as developmental growth and differentiation factors. Dev. Dyn. 237, 1537–1552 (2008).
Bastek, J. A. et al. Biomarkers of inflammation and placental dysfunction are associated with subsequent preterm birth. J. Matern. Fetal Neonatal Med. 24, 600–605 (2011).
Leviton, A. et al. Relationships among the concentrations of 25 inflammation-associated proteins during the first postnatal weeks in the blood of infants born before the 28th week of gestation. Cytokine 57, 182–90 (2012).
Wei, S. Q., Fraser, W. & Luo, Z. C. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet. Gynecol. 116, 393–401 (2010).
Lee, B. K. et al. Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain Behav. Immun. 44, 100–105 (2015).
Onore, C., Careaga, M. & Ashwood, P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav. Immun. 26, 383–392 (2012).
Rusterholz, C., Hahn, S. & Holzgreve, W. Role of placentally produced inflammatory and regulatory cytokines in pregnancy and the etiology of preeclampsia. Semin. Immunopathol. 29, 151–162 (2007).
Roescher, A. M. et al. Placental pathology and neurological morbidity in preterm infants during the first two weeks after birth. Early Hum. Dev. 90, 21–25 (2014).
Kramer, B. W. et al. Decreased expression of angiogenic factors in placentas with chorioamnionitis after preterm birth. Pediatr. Res. 58, 607–612 (2005).
Roescher, A. M. et al. Placental pathology, perinatal death, neonatal outcome, and neurological development: a systematic review. PLoS ONE 9, e89419 (2014).
Atladottir, H. O. et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J. Autism Dev. Disord. 40, 1423–1430 (2010).
Chess, S. Autism in children with congenital rubella. J. Autism Child Schizophr. 1, 33–47 (1971).
Guinchat, V. et al. Pre-, peri- and neonatal risk factors for autism. Acta Obstet. Gynecol. Scand. 91, 287–300 (2012).
Challier, J. C. et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 29, 274–281 (2008).
Roberts, K. A. et al. Placental structure and inflammation in pregnancies associated with obesity. Placenta 32, 247–254 (2011).
Ghidini, A. & Salafia, C. M. Sex differences of placental dysfunction in severe prematurity. BJOG 112, 140–144 (2005).
Limperopoulos, C. et al. Positive screening for autism in ex-preterm infants: prevalence and risk factors. Pediatrics 121, 758–765 (2008).
Straughen, J. K. et al. The association between placental histopathology and autism spectrum disorder. Placenta 57, 183–188 (2017).
Raghavan, R. et al. Preterm birth subtypes, placental pathology findings, and risk of neurodevelopmental disabilities during childhood. Placenta 83, 17–25 (2019).
Albers, C. A. & Grieve, A. J. Test review: Bayley, N. (2006). Bayley Scales of Infant and Toddler Development—Third Edition. San Antonio, TX: Harcourt Assessment. J. Psychoeduc. Assess. 25, 180–190 (2007).
Vohr, B. R. et al. Are outcomes of extremely preterm infants improving? Impact of Bayley assessment on outcomes. J. Pediatr. 161, 222–8.e3 (2012).
Robins, D. L. et al. The Modified Checklist for Autism in Toddlers: an initial study investigating the early detection of autism and pervasive developmental disorders. J. Autism Dev. Disord. 31, 131–144 (2001).
Lord, C. et al. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) [Manual: Modules 1-4] (Western Psychological Services, Torrance, 2012).
Chlebowski, C. et al. Using the childhood autism rating scale to diagnose autism spectrum disorders. J. Autism Dev. Disord. 40, 787–799 (2010).
Gardener, H., Spiegelman, D. & Buka, S. L. Prenatal risk factors for autism: comprehensive meta-analysis. Br. J. Psychiatry 195, 7–14 (2009).
Greer, L. G. et al. An immunologic basis for placental insufficiency in fetal growth restriction. Am. J. Perinatol. 29, 533–538 (2012).
Wintermark, P. et al. Placental pathology in asphyxiated newborns meeting the criteria for therapeutic hypothermia. Am. J. Obstet. Gynecol. 203, 579 e1–579 e9 (2010).
Redline, R. W. et al. Placental diagnostic criteria and clinical correlation—a workshop report. Placenta 26, S114–S117 (2005).
Mir, I. N. et al. Placental pathology is associated with severity of neonatal encephalopathy and adverse developmental outcomes following hypothermia. Am. J. Obstet. Gynecol. 213, 849 e1–7 (2015).
Redline, R. W. et al. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr. Dev. Pathol. 6, 435–448 (2003).
Redline, R. W. & Patterson, P. Villitis of unknown etiology is associated with major infiltration of fetal tissue by maternal inflammatory cells. Am. J. Pathol. 143, 473–479 (1993).
Redline, R. W. Villitis of unknown etiology: noninfectious chronic villitis in the placenta. Hum. Pathol. 38, 1439–1446 (2007).
Redline, R. W. et al. Maternal vascular underperfusion: nosology and reproducibility of placental reaction patterns. Pediatr. Dev. Pathol. 7, 237–249 (2004).
Redline, R. W. et al. Fetal vascular obstructive lesions: nosology and reproducibility of placental reaction patterns. Pediatr. Dev. Pathol. 7, 443–452 (2004).
Renaer, M., Van de Putte, I. & Vermylen, C. Massive feto-maternal hemorrhage as a cause of perinatal mortality and morbidity. Eur. J. Obstet. Gynecol. Reprod. Biol. 6, 125–140 (1976).
Lewis, N. E., Marszalek, L. & Ernst, L. M. Placental pathologic features in fetomaternal hemorrhage detected by flow cytometry. Pediatr. Dev. Pathol. 20, 142–151 (2017).
Pinar, H. et al. Reference values for singleton and twin placental weights. Pediatr. Pathol. Lab. Med. 16, 901–907 (1996).
Salafia, C. et al. Characterization of placental growth as a biomarker of Autism/Asd risk. Placenta 33, A16–A16 (2012).
Wu, Y. W. & Colford, J. M. Jr. Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA 284, 1417–1424 (2000).
Aoki, Y. et al. Association of white matter structure with autism spectrum disorder and attention-deficit/hyperactivity disorder. JAMA Psychiatry 74, 1120–1128 (2017).
Naldini, A. & Carraro, F. Role of inflammatory mediators in angiogenesis. Curr. Drug Targets Inflamm. Allergy 4, 3–8 (2005).
Favrais, G. et al. Systemic inflammation disrupts the developmental program of white matter. Ann. Neurol. 70, 550–565 (2011).
Felderhoff-Mueser, U. et al. Oxygen causes cell death in the developing brain. Neurobiol. Dis. 17, 273–282 (2004).
Ment, L. R. et al. Association of chronic sublethal hypoxia with ventriculomegaly in the developing rat brain. Brain Res. Dev. Brain Res. 111, 197–203 (1998).
Fleiss, B. et al. Inflammation-induced sensitization of the brain in term infants. Dev. Med. Child Neurol. 57, 17–28 (2015).
van Tilborg, E. et al. Combined fetal inflammation and postnatal hypoxia causes myelin deficits and autism-like behavior in a rat model of diffuse white matter injury. Glia 66, 78–93 (2018).
Stein, Z. & Susser, M. The Dutch Famine, 1944–1945, and the reproductive process. II. Interrelations of caloric rations and six indices at birth. Pediatr. Res. 9, 76–83 (1975).
Lumey, L. H. Compensatory placental growth after restricted maternal nutrition in early pregnancy. Placenta 19, 105–111 (1998).
Beischer, N. A., Holsman, M. & Kitchen, W. H. Relation of various forms of anemia to placental weight. Am. J. Obstet. Gynecol. 101, 801–809 (1968).
Thompson, L. P. et al. Placental hypoxia during early pregnancy causes maternal hypertension and placental insufficiency in the hypoxic guinea pig model. Biol. Reprod. 95, 128 (2016).
Kim, S. H. et al. Predictive validity of the Modified Checklist for Autism in Toddlers (M-CHAT) born very preterm. J. Pediatr. 178, 101–107. e2 (2016).
Luyster, R. J. et al. The Modified Checklist for Autism in Toddlers in extremely low gestational age newborns: individual items associated with motor, cognitive, vision and hearing limitations. Paediatr. Perinat. Epidemiol. 25, 366–376 (2011).
Acknowledgements
This work was supported by Departmental Funding from UT Southwestern Medical Center.
Author information
Authors and Affiliations
Contributions
All authors contributed to drafting the article or revising it critically for important intellectual content and final approval of the submitted version. Specifically, I.N.M. participated in concept, study design, sample and data acquisition and interpretation, statistical analysis and drafted the first version of the manuscript, and finalized the manuscript for submission after comments from the other authors. L.S.B. performed the statistical analysis, participated in data interpretation and review, revision of the manuscript, and reviewed the final version. S.P.W., R.H., C.R.R., and L.F.C. participated in concept, study design, data interpretation and review, revision of the manuscript, and participated in finalizing the manuscript after comments from the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Statement of consent
We had waiver of consent for this retrospective study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mir, I.N., White, S.P., Steven Brown, L. et al. Autism spectrum disorders in extremely preterm infants and placental pathology findings: a matched case–control study. Pediatr Res 89, 1825–1831 (2021). https://doi.org/10.1038/s41390-020-01160-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-01160-4
This article is cited by
-
Impact of maternal infection on outcomes in extremely preterm infants
Pediatric Research (2024)
-
The prevalence and profile of autism in individuals born preterm: a systematic review and meta-analysis
Journal of Neurodevelopmental Disorders (2021)
-
Placental origins of neonatal diseases: toward a precision medicine approach
Pediatric Research (2021)