Abstract
Background
Metabolic bone disease of prematurity (MBD) is a common problem among preterm infants. Our previous study identified cholestasis as an important risk factor for the development of MBD. We conducted this study to determine the vitamin D status in preterm infants with MBD and cholestasis.
Methods
We retrospectively reviewed medical record of preterm infants evaluated in NICU at Holtz Children’s/Jackson Memorial Hospital between June 2014 and May 2016. Demographic, biochemical data, and vitamin D intake were collected and analyzed.
Results
We identified 58 preterm infants (median gestational age 25 weeks) with MBD during this period. Twenty five infants also developed cholestasis. Median serum 25-hydroxyvitamin D level at the time of diagnosis of MBD was similar in cholestasis (C), (29.1 ng/ml, IQR 24.4–33.5), and non-cholestasis (NC), (28.7 ng/ml, IQR 22.7–34.6), group (p = 0.41). At the second measurement, average 6 weeks after the first measurement; median serum 25-hydroxyvitamin D level was lower (p = 0.02) in the C group (31.2 ng/ml, IQR 23.0–38.8) than in the NC group (36.5 ng/ml, IQR 28–45). However, the actual percentage of infants with vitamin D deficiency was similar in both the groups.
Conclusion
Most preterm infants with cholestasis and MBD had normal vitamin D status.
Similar content being viewed by others
Introduction
Metabolic bone disease of prematurity (MBD) is a common problem among preterm infants especially those with extremely low birth weight (<1000 g).1 The active transport of calcium and phosphorus via the placenta plays an important role in the mineralization of fetal skeletons. As much as 80% of total mineral transport occurs during the third trimester,2 and infants born prior to the completion of this process are at risk for MBD. Current management of bone health in preterm infants includes optimization of mineral and nutritional intakes via parenteral or enteral nutrition. We recently reported that preterm infants who developed MBD received similar amount of minerals compared to those without MBD.3 In particular, MBD still occurred in extremely premature infants who received optimal mineral intake. This suggested that comorbidities associated with preterm birth may play an additional role in the development of MBD. Our previous study indicated that preterm infants who developed cholestasis had the highest odds ratio (9.6, 95% confidence interval (CI) 2.1–45.3) of developing MBD even after adjusting for birth weight, duration of glucocorticoid use, and parenteral nutrition.3 Similar findings were reported by Lee et al.4 who reported that the risk of MBD was 18 times higher in preterm infants with cholestasis and also by Viswanathan et al.1 who reported that preterm infants with MBD had significantly higher serum direct bilirubin compared to those who did not develop MBD.
In preterm infants, cholestasis from parenteral nutrition-associated liver disease (PNALD) is a common complication of parenteral nutrition. It is estimated that 40–60% of neonates who require parenteral nutrition develop cholestasis5 and evidence of liver dysfunction may occur as early as 14 days after the start of parenteral nutrition.6 The absorption of vitamin D depends largely on the availability of bile acids in the intestines and several reports have shown that infants and children with cholestasis have subnormal serum 25-hydroxyvitamin D (25-OH vitamin D) levels.7,8,9 Furthermore, Jensen et al. reported difficulty in achieving normal serum 25-hydroxyvitmain D level in infants and children with cholestasis from different type of liver diseases despite receiving high-dose repletion therapy.10 Standard pediatric parenteral solutions provide multivitamins, including vitamin D. However, Wozniack et al. and Ubesie et al. reported a high prevalence of suboptimal vitamin D level in children who received parenteral nutrition for a prolonged period.11,12
Vitamin D plays an important role in mineral homeostasis and bone metabolism mainly through the regulation of intestinal transport of calcium and phosphorus. In children with long-standing vitamin D deficiency, parathyroid hormone (PTH) levels rise in response to the decline in serum calcium. This results in the mobilization of calcium from the bone and eventually rickets.13
These observations suggest that altered vitamin D homeostasis could have a role in the development of MBD in preterm infants with cholestasis. The goal of current study was to determine the role of vitamin D in bone health of preterm infants. To investigate this, we determined, vitamin D status and biochemical data in preterm infants who developed cholestasis and MBD.
Methods
We performed a retrospective chart review of preterm infants in the newborn intensive care unit at Holtz Children’s Hospital/Jackson Memorial Hospital from June 2014 to May 2016. The study was approved by the Institutional Review Board of the University of Miami, Miller School of Medicine.
Identification of preterm infants with MBD
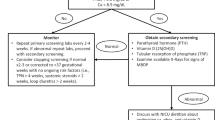
Since June 2014, we have been conducting weekly rounds to identify MBD in patients in the newborn intensive care unit at Holtz Children’s Hospital/Jackson Memorial Hospital. Screening tests were routinely performed at around 4–6 weeks postnatal age in preterm infants at risk for MBD, especially in those who were born at <30 weeks or with birth weight <1000 g. The screening tests included determination serum calcium, phosphorus, alkaline phosphatase (ALP), and examination of knee radiogram. In addition, we also obtained serum level of 25-OH vitamin D and PTH. MBD was classified as serum ALP > 500 IU/L and the presence of characteristic radiographic changes of the bone as previously described.3 Infants were subdivided into groups that included those with MBD assigned to cholestasis (C) and non-cholestasis (NC) groups. Cholestasis was defined as the persistence of serum direct bilirubin > 2 mg/dl for >2 weeks.
Treatment of preterm infant with MBD
After MBD cases were identified, the nutritional and mineral intakes were optimized. Fortification concentrate (Mead Johnson) was added to the mother’s breast milk. Premature infant formula (Enfamil, Mead Johnson) was used if mother did not produce adequate amount of breast milk. Fortification was added when the amount of breast milk intake reached 60 ml/kg/day. The amount of calcium in fortified breast milk and in premature formula was approximately 116 mg/dl and 132 mg/dl, respectively, and the amount of phosphorus was approximately 64 mg/dl and 66 mg/dl, respectively. The amount of vitamin D was approximately 168 units/dl in fortified breast milk and 240 units/dl in premature formula. Our previous data indicated that the amount of calcium and phosphorus intake was comparable in infants who developed and those who did not develop MBD3 and suggested that our feeding protocol was able to optimize the amount of mineral intake. If the infants were still receiving parenteral nutrition, the amount of minerals was optimized as permitted by the maximal concentration in the solution. The soluble form of multivitamin, which also contains vitamin D, was routinely added.
Infants whose serum 25-OH vitamin D level was <30 ng/ml received vitamin D supplement (400 units/day) if they were able to tolerate oral feedings. Infants with elevated serum PTH (>100 pg/ml) were administered an oral form of calcitriol (dose range 0.05–0.1 mcg/kg/day) for 4–6 weeks. This was done because calcitriol treatment has been shown to be effective in reducing PTH and decreasing urinary phosphate wasting in preterm infants with MBD.2
Follow-up studies
Serum calcium and phosphorus were monitored weekly in infants who were received full enteral feedings and twice weekly in infants who were receiving parenteral nutrition. The amount of formula or parenteral nutrition was weight adjusted. Determination of serum 25-OH vitamin D levels was performed a second time about 6 weeks after the first measurement.
Data collection
Demographic and biochemical data were collected at the initial diagnosis of MBD and from subsequent analyses. We collected data for both enteral and parenteral nutrition over the first 3 months of life by reviewing nutritional data on the last day of every 2-week period. We combined both parenteral and enteral intake data during the transition period to full enteral feeding in the analysis of total calcium, phosphorus, and vitamin D intake. The study timeline is illustrated in Fig. 1a.
a Study timeline of data collection and laboratory tests. b Boxplot of serum 25-hydroxyvitamin D (25-OH vitamin D) level. Median serum 25-OH vitamin D level at the time metabolic bone disease of prematurity was diagnosed was similar in cholestasis (C; 29.1 ng/ml, interquartile range (IQR) 24.4–33.5) and non-cholestasis (NC; 28.7 ng/ml, IQR 22.7–34.6) groups. Vitamin D level at the second measurement was higher (p = 0.04) in the NC (36.5 ng/ml, IQR 28.0–45.0) than in the C (31.2 ng/ml, IQR 23.5–38.8) group. c The proportion of infants with vitamin D deficiency was also similar in the C and NC groups both at first (C vs. NC; 12% vs. 13%, p value = 0.9) and second measurement (C vs. NC; 15% vs. 11%, p = 0.7)
Statistical analyses
Data were analyzed using the IBM SPSS software version 24. Mann–Whitney U test was used for data that did not have normal distribution and Independent samples T test was used for data with normal distribution. Two-way repeated-measures analysis of variance was used to analyze serial data of vitamin D intake. The statistical significance was analyzed for the interaction between group and time for vitamin D intake.
Results
Demographic data
We identified 58 preterm infants who developed MBD during the review period. The average postnatal age at the diagnosis was 6 weeks after birth. Twenty five (43%) infants also developed cholestasis. Median gestational age was similar (p = 0.9) in the C (25 weeks, IQR 24–26) and NC groups (25 weeks, IQR 24–26). However, median birth weight was significantly lower (p = 0.02) in C (545 g, IQR 375–715) compared to the NC group (675 g, IQR 504–846). Percentage of patients with intrauterine growth restriction (IUGR) was higher in the C (28%) than in the NC (10%) group but the difference did not reach the significant level (p = 0.59). However, the percentage of patients born to the mother with cervical insufficiency was significantly higher (p = 0.01) in the C (24%) than in the NC (3%) group. In addition, the percentage of patients born to mothers with pre-eclampsia was significantly higher (p = 0.02) in the C (32%) compared with the NC (9%) group. The frequency of other pregnancy-related complications, including chronic hypertension, reversed end-diastolic blood flow, preterm premature rupture of the membranes, and preterm labor was not significantly different between the C and NC groups (Table 1). The ratio of male to female infants was similar (p = 0.79) in the C (48%) and NC (51%) groups. Median duration of parenteral nutrition was significantly longer (p < 0.01) in the C (85 days, IQR 62.5–107.5) than it was in the NC (34 days, IQR 24.5–43.5) group. The percentage of patients who developed necrotizing enterocolitis (NEC) requiring a surgical intervention (Bell’s criteria >stage II) was also higher (p < 0.01) in the C (48%) than in the NC (15%) group. The percentage of patient who had septicemia also was slightly higher in the C (44%) than in the NC (24%) group but did not reach the significant level (p = 0.1).
When cholestasis occurred, it was diagnosed on the average 39 days after birth and typically persisted for 79.6 days. Median peak bilirubin level in the C group was 8.3 mg/dl (IQR 5.5–11) compared to 0.7 mg/dl (IQR 0.2–1.2 mg/dl) in the NC group (p < 0.01). These data are summarized in Table 1.
Vitamin D status and biochemical data
Median serum 25-OH vitamin D level (Fig. 1b) at the diagnosis of MBD was similar (p = 0.41) in the C (29.1 ng/ml, IQR 24.4–33.5) and NC (28.7 ng/ml, IQR 22.7–34.6) groups. At the second determination, median serum 25-OH vitamin D level was significantly higher (p = 0.04) in the NC (36.5 ng/ml, IQR 28.0–45.0) than in the C (31.2 ng/ml, IQR 23.5–38.8) group. Despite this, the percentage of infants with actual vitamin D deficiency (serum 25-OH vitamin D <20 ng/ml) was similar in both groups (Fig. 1c) both at the first (C vs. NC; 12% vs. 13%, p value = 0.9) and the second determination of serum 25-OH vitamin D (C vs. NC; 15% vs.11%, p = 0.7).
The average serum calcium level (mean ± SD) during the period prior to the first measurement of serum 25-OH vitamin D was similar (p = 0.54) in the C (9.5 ± 0.55 mg/dl) and NC (9.6 ± 0.85 mg/dl) groups. Similarly, the average serum phosphorus (mean ± SD) during that same time was similar (p = 0.07) in the C (5.1 ± 1.2 mg/dl) compared to that of the NC (5.7 ± 1.2 mg/dl) group. However, the average serum level of calcium during the 6-week period between first and second measurement was significantly lower (p < 0.01) in C (9.6 ± 0.92 mg/dl) than in NC (10.1 ± 0.49 mg/dl) group. In addition, the average serum level of phosphorus during the same period was significantly lower (p < 0.01) in the C group (5.5 ± 0.86 mg/dl) compared to that of the NC group (6.1 ± 1.2 mg/dl). Median serum PTH obtained at the diagnosis of MBD was slightly lower in the C (58.3 pg/ml, IQR 24.3–92.3) than in the NC (77 pg/ml, IQR 41.5–112.5) group but the difference was not statistically significant (p = 0.07). The data are shown in Table 2.
Vitamin D and mineral intakes
Vitamin D intake is shown in Fig. 2 and mineral intake in Fig. 3. The average (mean ± SD) vitamin D intake from combined enteral and parenteral routes 4 weeks prior, 2 weeks prior, and on the week of the first serum 25-OH vitamin D measurement in subjects in the C group was 40.9 ± 9.1, 87.4 ± 145.7, and 140.5 ± 145.4 units/day, respectively, whereas that of subjects in the NC group was 82.9 ± 127.5, 271.9 ± 257.3, and 467.1 ± 238.1 units/day, respectively (Fig. 2a). The time × group effect of average vitamin D intake was significantly lower (p < 0.01) in the C compared to that in the NC group. During this period, the average vitamin D intake from parenteral nutrition in the C group was 40.8 ± 9.2, 37.5 ± 27.9, and 36.19 ± 37.4 units/day, which was significantly higher (time × group effect p value = 0.02) than that in the NC group whose average vitamin D intake from parenteral nutrition was 43.8 ± 26.1, 23.6 ± 32.06, and 6.6 ± 17.26 units/day, respectively (Fig. 2c). At the time of diagnosis of MBD, 18 patients (72%) of the C group were still receiving parenteral nutrition compared to only 6 patients (18%) in the NC group. The average (mean ± SD) vitamin D intake at 2, 4, and 6 weeks after the first measurement in the C group was 435.8 ± 300.7, 550.6 ± 432.7, and 731.0 ± 444.1 units/day, respectively, whereas that of NC group was 768.3 ± 380.1, 917.8 ± 328.5, and 940.0 ± 342.7 units/day, respectively (Fig. 2b). The time × group effect was not significantly different (p = 0.28) between the C and NC groups. The average vitamin D intake from parenteral nutrition during this period in the C group was 65.2 ± 85.6, 38.8 ± 66.5, and 32.5 ± 70.4 units/day, respectively (Fig. 2d). Only 3 patients in the NC group received parenteral nutrition in the first 2-week period after the diagnosis of MBD and none received parenteral nutrition beyond this 2-week period.
Comparison between total vitamin D intake prior to (a) and after (b) the first measurement of serum 25-hydroxyvitamin D between the cholestasis and non-cholestasis groups by two-way repeated-measures analysis of variance. The average vitamin D intake was higher in the non-cholestasis group than in the cholestasis group prior to the first measurement but was then comparable during the period after first measurement. By contrast, vitamin D intake from parenteral nutrition was significantly lower in the non-cholestasis group at both study periods (c, d) indicating that most of vitamin D intake in this group was from oral feedings
Comparison between total calcium intake via both enteral and parenteral route (a), parenteral route alone (b), and phosphorus intake via both enteral and parenteral route (c), and parenteral route (d) after the first measurement of serum 25-hydroxyvitamin D between the cholestasis and non-cholestasis groups by two-way repeated-measures analysis of variance. The amount of calcium and phosphorus intakes were similar (p = 0.67 and 0.7, respectively) in both the groups
With respect to mineral intake, the average (mean ± SD) total amount of calcium intake for both enteral and parenteral route, determined at 2, 4, and 6 weeks, after the first vitamin D measurement was 106.5 ± 59.5, 115.71 ± 64.5, and 132.5 ± 67.6 mg/kg/day, respectively, in the C group and 137.3 ± 52.9, 161 ± 48.3, and 164.1 ± 42.2 mg/kg/day, respectively, in the NC group (Fig. 3a). The average amount over this period (time × group effect) was not statistically significant (p = 0.67). The amount of calcium intake from parenteral nutrition during this period was 30.1 ± 30.5, 28.4 ± 32, and 25 ± 35.7 mg/kg/day, respectively, in the C group (Fig. 3b). The average amount of total phosphorus intake, received via enteral and parenteral during this period was 62 ± 29.8, 70.8 ± 41, and 81.2 ± 42 mg/kg/day, respectively, in the C group and 73.8 ± 29.1, 86.6 ± 26.1, and 88.4 ± 22.8 mg/kg/day, respectively, in the NC group (Fig. 3c). The time × group effect of the total phosphorus intake was not significant (p = 0.7) between the two groups. The average phosphorus intake via parenteral route during this period was 30.1 ± 30.6, 28.4 ± 32.1, and 25 ± 35.7 mg/kg/day, respectively, in the C group (Fig. 3d). Only three patients in NC group received parenteral nutrition until 2 weeks after the first vitamin D measurement, whereas the rest received full enteral feedings by the time the first serum vitamin D level was obtained.
Discussion
The principle focus of this study was the investigation of the role of vitamin D deficiency in the development of MBD. As our previous work implicated cholestasis as an important predictor of MBD, we also sought to determine whether cholestasis increased the risk for vitamin D deficiency in preterm infants. Among the 58 infants in our study, only 8 (13.8%) had vitamin D deficiency. As routine determination of serum 25-hydroxyvitamin D level is not standard of care in preterm infants, we cannot estimate the prevalence of vitamin D deficiency among preterm infants without MBD. Nonetheless, the small number of preterm infants with MBD and vitamin D deficiency suggests that vitamin D deficiency does not play a major role in MBD.
The observation that cholestasis occurred in almost half of preterm infants with MBD in our report was in agreement with our previous observation and other groups,3,4 that cholestasis was a prevalent co-morbidity in preterm infants who develop MBD. PNALD was the presumed cause of cholestasis in all cases as other possible causes such as biliary atresia, viral infections such as cytomegalovirus, or alpha-1 antitrypsin were excluded. A higher rate of intestinal surgery for NEC and a longer duration parenteral nutrition was observed in the C group, consistent with other reports that these conditions are significant factors for the development of PNALD.14,15 Sepsis has also been identified as a risk factor for cholestasis in preterm infant.16 However, the rate of septicemia/sepsis in our study was similar between the C and NC groups, suggesting that sepsis was not an important confounding factor to the development of cholestasis in our report. Although patients in the C group had significantly lower birth weight than those in the NC group, the percentage of infants that met the criteria for IUGR was not different in the two groups. In a report by Alkharfy et al., lower birth weight was a significant predictive risk factor for cholestasis in addition to the duration of parenteral nutrition.17 Our results support the observation that lower birth weight, independent of IUGR status, creates risk for cholestasis in extreme preterm infants.
Serum vitamin D levels were similar in the C and NC group at the first measurement. Although the median serum 25-OH vitamin D level in NC group was significantly higher at the second measurement, the median serum 25-OH vitamin D level in C group was >30 ng/ml, a value considered a sufficient level. Furthermore, there was no difference in the prevalence of vitamin D deficiency between preterm infants with cholestasis compared to those without cholestasis at both measurements. These findings suggested that vitamin D homeostasis was preserved in extremely preterm infants whose nutritional intake was optimized despite the occurrence of cholestasis.
It is noteworthy that the amount of vitamin D intake prior to the first determination of 25-OH vitamin D measurement was significantly less in the C group than in the NC group even though patients with cholestasis had similar serum 25-OH vitamin D level at the first determination. During the period between tests, preterm infants with cholestasis received most of vitamin D through parenteral nutrition, and it is possible that the provision of vitamin D through parenteral route was effective to maintain serum 25-OH vitamin D level. Although the amount of vitamin D delivered was less, vitamin D does not require intestinal absorption. In a report by Koo et al., preterm infants with an average weight of 1089 g were provided vitamin D parenterally and were able to maintain normal serum 25-OH vitamin D levels despite receiving small amounts of vitamin D, 31–33 IU/day.18 By contrast, Kimura et al. reported that older children required higher amount of vitamin D, between 200 and 360 IU/day, through parenteral nutrition in order to achieve sufficient serum 25-OH vitamin D level.19
The discordance in vitamin D requirement through the parenteral route between infants and older children may be explained by the difference in the amount of peripheral fat tissue. Worstman et al. reported the decreased bioavailability of vitamin D in obesity and the response to oral vitamin D intake is inversely correlated with body mass index.20 Gangloff et al. later reported the correlation between loss of adipose tissue and an increase in serum 25-OH vitamin D level.21 Preterm infants typically have much less fat tissue compared to infants born at term and therefore less peripheral stores of vitamin D but higher bioavailable vitamin D in the circulation.
Although cholestasis did not have a significant effect on the prevalence of vitamin D deficiency, it did appear to have some effects on the absorption of vitamin D as serum level of 25-OH vitamin D level at the second determination in the C group was lower than that in the NC group, this despite having received similar amounts of vitamin D. In addition, the higher levels of serum calcium and phosphorus observed in the NC group despite intakes of similar amount of calcium and phosphorus could also have been a reflection of higher level of serum 25-OH vitamin D. Chhina et al. reported a positive correlation between serum ionized calcium, phosphorus, and serum 25-OH vitamin D level in preterm infants.22 In our study, we were not able to determine whether the difference in serum calcium and phosphorus between the C and NC groups had any impact on the healing of MBD or bone mineral content. Additional studies are needed to investigate this.
Moreira et al. reported elevated PTH levels in 85% of extremely low birth weight infants with radiographic evidence of MBD and subsequent reduction in PTH among subjects who received high-dose oral calcium carbonate.23 In our study, preterm infants in the NC group had higher but not statistically significant levels of serum PTH at the diagnosis of MBD when compared to those in the C group despite similar levels of serum 25-OH vitamin D, calcium, and phosphorus. This suggests that the abnormally elevated PTH levels in preterm infants with MBD might not be the result of alterations in mineral or vitamin D homeostasis. Other factors such as direct stimulatory effect on the secretion of PTH from high phosphorus content in preterm formula or less renal responsiveness to PTH may be responsible for the elevated serum PTH level frequently observed in preterm infants.
In conclusion, our study indicated that vitamin D deficiency is unlikely to play a major role in the development of MBD in preterm infants with cholestasis. Other factors such as direct toxicity of bilirubin and bile acids on osteoblast, as previously shown in studies by Ruis-Gaspa et al.,24,25 may play a larger role in the development of MBD. Even though frank vitamin D deficiency may not be an important factor in the etiology of MBD, it remains important to ensure adequate provision of vitamin D to preterm infants to ensure optimal mineral homeostasis. Vitamin D supplementation should be done in a judicious manner and with close monitoring to avoid vitamin D toxicity. Indeed hypervitaminosis D was found in 19% and 75% of preterm infants receiving preterm formula and vitamin D supplement in reports by Kolodziejczyk et al.26 and Nako et al.,27 respectively.
Limitations in our study include a relatively small sample size and the absence of information of maternal vitamin D, supplementation, or vitamin D levels that may have influenced the level of serum 25-OH vitamin D of their infants. We also did not have cord blood level of 25-OH vitamin D prior to the diagnosis of cholestasis or MBD. Determining the change in serum 25-OH vitamin D level before and after the development of cholestasis may provide an additional evidence on the effect of cholestasis on vitamin D status of these infants.
References
Viswanathan, S. et al. Metabolic bone disease: a continued challenge in extremely low birth weight infants. J. Parenter. Enter. Nutr. 38, 982–990 (2014).
Rustico, S. E., Calabria, A. C. & Garber, S. J. Metabolic bone disease of prematurity. J. Clin. Transl. Endocrinol. 1, 85–91 (2014).
Ukarapong, S., Venkatarayappa, S. K. B., Navarrete, C. & Berkovitz, G. Risk factors of metabolic bone disease of prematurity. Early Hum. Dev. 112, 29–34 (2017).
Lee, S. M. et al. High incidence of rickets in extremely low birth weight infants with severe parenteral nutrition-associated cholestasis and bronchopulmonary dysplasia. J. Korean Med. Sci. 27, 1552–1555 (2012).
Hojsak, I. et al. ESPGHAN Committee on Nutrition Position Paper. Intravenous lipid emulsions and risk of hepatotoxicity in infants and children: a systematic review and meta-analysis. J. Pediatr. Gastroenterol. Nutr. 62, 776–792 (2016).
Christensen, R. D., Henry, E., Wiedmeier, S. E., Burnett, J. & Lambert, D. K. Identifying patients, on the first day of life, at high-risk of developing parenteral nutrition-associated liver disease. J. Perinatol. 27, 284–290 (2007).
Heubi, J. E., Hollis, B. W., Specker, B. & Tsang, R. C. Bone disease in chronic childhood cholestasis. I. vitamin D absorption and metabolism. Hepatology 9, 258–264 (1989).
Mohammadi, B. et al. Prevalence of vitamin D deficiency and rickets in children with cholestasis in Iran. Acta Med. Iran 50, 482–485 (2012).
Samra, N. M., Emad El Abrak, S., El Dash, H. H., El Said El Raziky, M. & El Sheikh, M. A. Evaluation of vitamin D status bone mineral density and dental health in children with cholestasis. Clin. Res. Hepatol. Gastroenterol. 42, 368–377 (2018).
Jensen, M., Abu-El-Haija, M., Bishop, W. & Rahhal, R. M. Difficulty achieving vitamin D sufficiency with high-dose oral repletion therapy in infants with cholestasis. J. Pediatr. Gastroenterol. Nutr. 61, 187–189 (2015).
Wozniak, L. J., Bechtold, H. M., Reyen, L. E., Hall, T. R. & Vargas, J. H. Vitamin D deficiency in children with intestinal failure receiving home parenteral nutrition. JPEN J. Parent. Enter. Nutr. 39, 471–475 (2015).
Ubesie, A. C. et al. Vitamin D deficiency and low bone mineral density in pediatric and young adult intestinal failure. J. Pediatr. Gastroenterol. Nutr. 57, 372–376 (2013).
Moon, R. J., Harvey, N. C., Davies, J. H. & Cooper, C. Vitamin D and skeletal health in infancy and childhood. Osteoporos. Int. 25, 2673–2684 (2014).
Duro, D. et al. Risk factors for parenteral nutrition-associated liver disease following surgical therapy for necrotizing enterocolitis: a Glaser Pediatric Research Network Study [corrected]. J. Pediatr. Gastroenterol. Nutr. 52, 595–600 (2011).
Koseesirikul, P., Chotinaruemol, S. & Ukarapol, N. Incidence and risk factors of parenteral nutrition-associated liver disease in newborn infants. Pediatr. Int. 54, 434–436 (2012).
Satrom, K. & Gourley, G. Cholestasis in preterm infants. Clin. Perinatol. 43, 355–373 (2016).
Alkharfy, T. M., Ba-Abbad, R., Hadi, A., Sobaih, B. H. & AlFaleh, K. M. Total parenteral nutrition-associated cholestasis and risk factors in preterm infants. Saudi J. Gastroenterol. 20, 293–296 (2014).
Koo, W. W. et al. Minimal vitamin D and high calcium and phosphorus needs of preterm infants receiving parenteral nutrition. J. Pediatr. Gastroenterol. Nutr. 8, 225–233 (1989).
Kimura, S. et al. Serum levels of vitamin D metabolites in children receiving total parenteral nutrition. JPEN J. Parent. Enter. Nutr. 10, 191–194 (1986).
Wortsman, J., Matsuoka, L. Y., Chen, T. C., Lu, Z. & Holick, M. F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 72, 690–693 (2000).
Gangloff, A. et al. Effect of adipose tissue volume loss on circulating 25-hydroxyvitamin D levels: results from a 1-year lifestyle intervention in viscerally obese men. Int. J. Obes. (Lond.) 39, 1638–1643 (2015).
Chhina, A. S., Shenoi, A., Nagendra, N., Rao, N. & Raja, M. Vitamin D and metabolic bone parameters in preterm neonates. Indian Pediatr. 53, 1023–1024 (2016).
Moreira, A. et al. Parathyroid hormone as a marker for metabolic bone disease of prematurity. J. Perinatol. 34, 787–791 (2014).
Ruiz-Gaspà, S. et al. Effects of bilirubin and sera from jaundiced patients on osteoblasts: Contribution to the development of osteoporosis in liver diseases. Hepatology 54, 2104–2113 (2011).
Ruiz-Gaspà, S. et al. Lithocholic acid downregulates vitamin D effects in human osteoblasts. Eur. J. Clin. Investig. 40, 25–34 (2010).
Kolodziejczyk, A. & Borszewska-Kornacka, M. K. Monitored supplementation of vitamin D in preterm neonates–a primary report. Dev. Period Med. 19(3 Pt 1), 313–318 (2015).
Nako, Y., Tomomasa, T. & Morikawa, A. Risk of hypervitaminosis D from prolonged feeding of high vitamin D premature infant formula. Pediatr. Int. 46, 439–443 (2004).
Author information
Authors and Affiliations
Contributions
S.U., W.Z. and C.N. collected and analyzed the data and drafted the manuscript. T.S. and G.B. extensively revised and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ukarapong, S., Zegarra, W., Navarrete, C. et al. Vitamin D status among preterm infants with cholestasis and metabolic bone disease. Pediatr Res 86, 725–731 (2019). https://doi.org/10.1038/s41390-019-0501-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0501-x
This article is cited by
-
Effect of early preventive supplementation with calcium and phosphorus on metabolic bone disease in premature infants
BMC Pediatrics (2024)
-
Establishment of a nomogram model for predicting metabolic bone disease in preterm infants: A case‒control study
European Journal of Pediatrics (2023)