Abstract
Objective
To evaluate vitamin D status in very low birth weight (VLBW) infants and response to vitamin D intake.
Study design
In this prospective cohort study of VLBW infants, 25-hydroxyvitamin D [25(OH)D] was measured regularly starting at birth. Daily vitamin D intake was estimated from parenteral and enteral sources.
Results
Of the included 83 infants born between November 2016 and March 2018, 44 (53%) had 25(OH)D < 30 ng/mL at birth but achieved vitamin D sufficiency (VDS) by 3 weeks while receiving 120–400 IU/day. Twenty-three (27.7%) infants had at least one 25(OH)D level >100 ng/mL during the study period. Infants whose intake was > 600 IU/day had higher prevalence of vitamin D excess (VDE).
Conclusion
In our study, low 25(OH)D was common in VLBW infants at birth. Vitamin D intake of 120–260 IU/day from parenteral and 200–400 IU/day from enteral route was appropriate for VLBW infants to achieve VDS. Doses > 600 IU/day increased risk of VDE.
Similar content being viewed by others
Introduction
Vitamin D, a fat-soluble vitamin, is critical for bone health and plays a crucial role in calcium and phosphorus metabolism. The discovery of vitamin D receptors in other organs suggests that the role of vitamin D extends beyond bone homeostasis [1,2,3]. Vitamin D deficiency (VDD) may increase the risk of respiratory tract infections, asthma, seizures, and growth disturbances [4,5,6]. Furthermore, animal studies support the hypothesis that VDD might alter the risk of bronchopulmonary dysplasia (BPD) [7, 8].
Vitamin D is primarily transferred to the fetus in the third trimester of pregnancy. Consequently, preterm infants are born with lower vitamin D levels [9,10,11]. Vitamin D levels at birth are also affected by race and season of birth. In the United States, African American women have the highest prevalence of VDD and are also at the greatest risk of premature delivery. As such, infants born to African American women tend to have lower vitamin D levels at birth and may be at a greater risk of its metabolic consequences [12,13,14].
As per the Endocrine Society, VDD is defined as a serum 25-hydroxyvitamin D [25(OH)D] below 20 ng/mL, insufficiency as a 25(OH)D of 20–29 ng/mL, and sufficiency as a 25(OH)D of 30–100 ng/mL. A 25(OH)D > 100 ng/mL is considered vitamin D excess (VDE) [3, 15]. The recommended daily vitamin D requirement for premature infants also varies. Current recommendation for oral supplementation varies from 200–400 IU/day as per the American Academy of Pediatrics (AAP) to 800–1000 IU/day by the European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) [16, 17]. Previous randomized trials of premature infants receiving daily vitamin D intake of 200, 400, 800, or 2000 IU reported varying levels of vitamin D sufficiency (VDS) [10, 11, 18,19,20,21]. Moreover, there is a scarcity of studies and guidelines addressing the vitamin D contents of parenteral nutrition for very low birth weight infants (VLBW, birth weight <1500 g). The American Society for Parenteral Nutrition guidelines suggests supplementing 200 IU of vitamin D per day [22]. The amounts of vitamin D in parenteral nutrition that can be provided are dependent on manufacturer multivitamin formulation, which is 120 IU for infants weighing <1 kg, and 260 IU for infants weighing between 1 and 3 kg. To our knowledge, the adequacy of this dose for VLBW infants has not been studied recently.
Thus, we sought: (1) to determine the prevalence of VDD, and insufficiency at birth in VLBW infants admitted to the neonatal intensive care unit (NICU) of an urban Chicago public hospital, and (2) to assess whether the current practice of vitamin D supplementation is appropriate for VLBW infants in our population.
Methods
Design
A prospective cohort study was conducted at the NICU of John Stroger, Jr. Hospital of Cook County, Chicago, Illinois. VLBW infants (inborn and outborn), born between November 1, 2016 and March 31, 2018 and admitted to NICU, were enrolled after obtaining informed written consent from their mothers. Infants who died within 7 days of life, or those with congenital anomalies or known inborn metabolic disorders were excluded from this study. The Institutional Review Board of Cook County Health System approved the study.
Vitamin D, calcium, and phosphorus intake
VLBW infants were started on parenteral nutrition from the first day of life. Enteral feeding was initiated with 10–20 mL/kg of maternal breast milk or regular premature formula (Enfamil® Premature 20 Cal/oz, Mead Johnson) within 24–48 h when the clinical condition was stable. Feeding was advanced at 10–20 mL/kg/day if tolerated at the discretion of the primary neonatologists. Donor human milk was not available at our center. Feeds were fortified to 24 Cal/oz using bovine milk-derived human milk fortifier (Enfamil Human Milk Fortifier Acidified Liquid, Mead Johnson) when infants’ enteral intake was more than 60 mL/kg/day [23]. If infants showed intolerance with new transition, fortified breast milk 22 Cal/oz was given for 1–2 days before advancing to 24 Cal/oz. Parenteral nutrition was gradually tapered as enteral nutrition was advanced and was discontinued when infants were receiving 120 mL/kg/d from feeds. Full volumes of enteral feedings were defined as infants attaining 150–160 mL/kg/day. Higher caloric formula 27 Cal/oz was prepared by combination of Enfamil Premature 24 Cal/oz and Enfamil premature 30 Cal/oz at 1:1 ratio and given to infants who required more calories or were fluid restricted. Infants weighing more than 1800–2000 g and showing steady weight gain were switched to either human milk without fortifier or transitional formula (Enfamil EnfaCare 22 Cal/Oz, Mead Johnson) 2–3 days before discharge from NICU.
Vitamin D in total parenteral nutrition was supplied at a dose of 120 IU/day (Infuvite Pediatric, Baxter) for infants weighing less than 1 kg, and 260 IU/day for infants between 1 and 3 kg. An oral multivitamin drop (Enfamil Poly-Vi-Sol) containing 400 IU of cholecalciferol was administered to infants when they no longer received parenteral nutrition and were older than 14 days of age.
The daily intake of vitamin D, calcium (Ca), and phosphorus (P) was averaged weekly over 1 week during the first 4 weeks of age and biweekly after 4 weeks of age. It was calculated using concentrations in parenteral nutrition, enteral feeds, and oral multivitamin supplementation based on recorded intake.
The concentration of vitamin D, Ca, and P in human milk and formulas was estimated as follows [23,24,25]:
Unfortified human milk 100 mL: vitamin D 7 IU, Ca 25 mg, P 15 mg
Fortified human milk 22 Cal/oz 100 mL: vitamin D 93 IU, Ca 75 mg, P 42 mg
Fortified human milk 24 Cal/oz 100 mL: vitamin D 167 IU, Ca 118 mg, P 65 mg
Premature formula 20 Cal/oz 100 mL: vitamin D 200 IU, Ca 112 mg, P 61 mg
Premature formula 24 Cal/oz 100 mL: vitamin D 240 IU, Ca 134 mg, P 73 mg
Premature formula 27 Cal/oz 100 mL: vitamin D 270 IU, Ca 150 mg, P 82 mg
Transitional formula 22 Cal/oz 100 mL: vitamin D 56 IU, Ca 89 mg, P 49 mg
Screening methods
Serum 25(OH)D concentration is considered the best marker of vitamin D status. Blood samples for 25(OH)D measurements were collected at birth, weekly until the infants were 4 weeks old, and then biweekly until they were 10 weeks old or were discharged from NICU, whichever came first. Infants who developed VDE at any point had their enteral multivitamin drops discontinued and serum 25(OH)D levels followed until levels normalized, or the infants were discharged from NICU.
Serum 25(OH)D levels were analyzed at the hospital laboratory on the day of blood sampling by using an Access 25(OH) Vitamin D Total assay (UniCel DxI 800 Access Immunoassay System, Beckman Coulter). The Access 25(OH) Vitamin D Total assay is a paramagnetic particle, chemiluminescent immunoassay for the quantitative determination of total 25(OH)D concentrations (a mixture of 25(OH)D2 and 25(OH)D3, which represents the best analytes for overall vitamin D status). The limit of detection of this assay is 2 ng/mL. The reportable measuring range is 7–120 ng/mL. Values outside of the reportable range were reported as < 7 or >120 ng/mL. The laboratory performed calibration every 28 days and quality control daily. The inter- and intra-assay coefficients of variation were 4.9–9%.
During this period, it was our NICU protocol to measure serum calcium, phosphorus, and alkaline phosphatase (ALP) in all VLBW infants biweekly until the infants were receiving full enteral feeds and weighed greater than 1800 g.
The Endocrine Society’s definition was adopted for this study. VDD was defined as a serum 25(OH)D below 20 ng/mL, vitamin D insufficiency (VDI) as a 25(OH)D of 20–29 ng/mL, VDS as a 25(OH)d of 30–100 ng/mL, and a 25(OH)D > 100 ng/mL was considered VDE [15]. Hypercalcemia was defined as serum Ca >11 mg/dL. Hypophosphatemia was defined as serum phosphorus level <4.5 mg/dL. Urine was also collected biweekly for assessment of tubular reabsorption of phosphate (TRP).
Data collection
Maternal demographic data: race, ethnicity, parity, the status of prenatal vitamin intake extracted from maternal chart, the season of birth, whether singleton or multiple births.
Neonatal data: gestational age (GA), birth weight, sex, Apgar scores, total daily parenteral and enteral intake of vitamin D, calcium, and phosphorus, diagnosis of respiratory distress syndrome (RDS), necrotizing enterocolitis (NEC), intraventricular hemorrhage (IVH), retinopathy of prematurity (ROP), and BPD. GA (in weeks) was calculated at birth based on an obstetrical estimate using the last menstrual period or first-trimester ultrasound scans. Appropriate for gestational age (AGA) was defined as birth weight between 10th and 90th percentile according to Fenton growth charts and small for gestational age (SGA) as a birth weight less than 10th percentile for GA [26]. NEC was diagnosed using modified Bell’s criteria based on clinical signs/symptoms and radiological findings [27]. Laser therapy for ROP was performed based on the recommendation from the Early Treatment of Retinopathy of Prematurity Study [28]. The severity of IVH was graded using Volpe’s Grading System [29]. Moderate to severe BPD was diagnosed if infants <32 weeks’ gestation needed O2 supplementation, and/or continuous positive airway pressure, or ventilator support at postmenstrual age 36 weeks [30].
Outcomes measurement
The primary outcome was serum 25(OH)D concentrations of infants at birth and during the NICU stays. The secondary outcome measurements were daily vitamin D intake, the response of 25(OH)D concentrations to vitamin D intake, and the relationship between serum calcium, phosphorus, ALP, and TRP with serum 25(OH)D concentrations.
Statistical analysis
Statistical analyses were performed utilizing SPSS Statistics 20. The Pearson correlation coefficient was used to study the relationship between vitamin D levels at birth and GA, birth weight. Independent sample t-tests, or ANOVA was used when comparing mean vitamin D levels in infants with different clinical characteristics. The clinical characteristics identified as being significant for lower vitamin D levels were retained for multivariable linear regression. χ2 tests or Fisher’s exact tests (when sample size ≤ 5) were used when comparing the prevalence of VDD at birth. A binary logistic regression model with serum 25(OH)D concentration < 20 ng/mL as an outcome variable was constructed to adjust GA and other potential variables. P Values of < 0.05 were considered significant.
Results
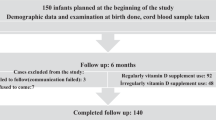
Of the subjects considered for inclusion, 94 VLBW infants were eligible for participation in this study, 5 infants died within 7 days of life, 4 did not have baseline 25(OH)D levels, and 2 parents did not provide consent. Complete data for 83 infants were available for analysis (Fig. 1). Maternal and infant characteristics and serum 25(OH)D levels at birth are shown in Table 1. Fifty-seven (69%) mothers were African American, 74 (89%) reported taking prenatal vitamins during pregnancy, and 65 (83%) received antenatal steroids. The mean GA of infants was 29.1 weeks (standard deviation [SD] 2.8; range 23–35); 30 (36%) were born ≤ 28 weeks of gestation. Mean birth weight was 1077 g (SD 254; range 550–1500); 32 (39%) infants were < 1000 g at birth, and 23 (28%) were SGA. Three infants died. Sixty-five infants were discharged home between 3 and 8 weeks of age. Fifteen infants remained in the NICU at 10 weeks of age.
GA and birth weight were positively correlated with 25(OH) D level (r = 0.447, p < 0.001; r = 0.23, p = 0.03, respectively). The distribution of serum vitamin D levels at birth of the 83 study infants is shown in Fig. 2. The mean serum 25(OH)D level at birth was 31.4 ng/mL (SD 13.8; range 7–61). Infants who were born ≤28 weeks of gestation, or AGA were more likely to have lower vitamin D levels than their counterparts (p < 0.001, p = 0.02, respectively) (Table 1). However, after multivariable linear regression, only GA remained significant (aOR 10.95, CI 5.29–16.62, p < 0.001).
At birth, 15 (18%) infants had VDD, 29 (35%) had VDI, and 39 (47%) were VDS (Table 2). Compared with infants born at >28 weeks of gestation, infants born ≤28 weeks had a significantly higher prevalence of VDD (40% vs. 5.7%, p < 0.001) and lower prevalence of VDS (20% vs. 62%, p < 0.001). AGA infants were also at lower prevalence of VDS than SGA infants (37% vs. 74%, p < 0.01). Compared with infants weighing 1000–1500 g, infants <1000 g did not have higher prevalence of VDD and VDI (p = 0.90). We found that infants with VDD and VDI combined had higher rate of RDS than infants with VDS (59% vs. 41%, p < 0.01). We did not find differences in the prevalence of low Apgar score ≤5 in infants with or without VDS.
Table 3 shows the vitamin D intake of the study population from all sources, including parenteral nutrition, feeds, and enteral multivitamin supplementation. All the infants except one received parenteral nutrition from the first day of life. The average daily vitamin D intake from enteral feeding during the first week was low due to smaller feeding volumes and negligible amounts of vitamin D in unfortified breast milk. Thus, extremely low birth weight infants (ELBW, birth weight <1000 g) received vitamin D almost exclusively from parenteral nutrition during the first 2 weeks of life, while most infants weighing ≥1000 g received their vitamin D mainly from parenteral nutrition during the first week of life. As infants grew older, enteral intake was increased; by the 4th week, the total vitamin D intake reached a median of 201 IU/day for infants <1000 g and 518 IU/day for infants ≥1000 g. By 6–8 weeks of life, intake reached 600 IU/day for most infants. Fifteen infants had a vitamin D intake of 800–1100 IU by 4 weeks of age when enteral multivitamin was being given (see also Table 4). Of note, the total intake of vitamin D decreased between the 6th and 10th weeks of life in bigger infants because the enteral supplementation of multivitamin was discontinued when the infants were found to have 25(OH)D > 100 ng/mL or when infants were switched to unfortified human milk/or transitional formula before discharge from NICU.
Mean 25(OH)D level in the study infants increased steadily from 31.4 ng/mL (SD 13.8) at birth to 99.3 ng/mL (SD 18.0) at 10 weeks of age (Supplementary Fig. 1). We stratified the study population into five sub-groups based on vitamin D intake and compared their concurrent vitamin D status during the NICU course (Table 4). The prevalence of VDD decreased from 18.1% at birth to 1.2% at 1 week and 0% at 2 weeks. The prevalence of VDI decreased from 34.9% at birth to 19.3% at 1 week and 2.4% at 2 weeks. All achieved VDS by the end of the third week while receiving vitamin D 120–400 IU/day. None of the infants were treated with any extra vitamin D when VDD or VDI was detected.
The prevalence of VDE increased as the infants gained weight and received more enteral nutrition, and consequently, more vitamin D. Prevalence of VDE increased from 20% at 6 weeks to 58.3% at 10 weeks. Overall, 23 (27.7%) infants had at least one 25(OH)D level >100 ng/mL during the study period. Infants whose vitamin D intake >600 IU/day had significantly higher rate of VDE than those taking ≤600 IU/day (20/79 = 25.3% vs. 17/346 = 4.9%, p < 0.001). None of these infants had documented clinical signs of vitamin D toxicity or hypercalcemia. The concurrent serum Ca levels were 8.8–10.6 mg/dL; serum P levels were 3.2–7.1 mg/dL; and ALP levels were 211–829 U/L.
Twenty percent (17/83) of infants had at least one episode of low phosphorus levels <4.5 mg/dL. The concurrent serum 25(OH)D levels were 33–120 ng/mL; Ca levels ranged from 9 to 11.9 mg/dL; ALP levels were 105–976 IU/L, and TRP were 0.55–0.99. Compared to infants with normal serum phosphorus, infants with hypophosphatemia had significantly higher serum ALP and lower calcium and phosphate intake when hypophosphatemia was detected. There was no significant difference in serum concentrations of Ca, 25(OH)D, or TRP between the two groups. Two infants developed osteopenia of prematurity with fractures during their NICU course. They were born at 23 and 25 weeks of gestation, weighed 570 and 620 g at birth, respectively. One had a diagnosis of NEC and was on prolonged parenteral nutrition; the other one was fed with low calcium and low phosphorus content elemental formula due to feeding intolerance. Both had low serum P levels (<4.5 mg/dL) and high ALP (>800 IU/L). One was noted to have low bone mineralization and rib fracture at 15 weeks, and the other was diagnosed with a healing fracture at 20 weeks old. Their serum Ca levels were in normal range. Both had sufficient to excess vitamin D levels (ranged from 36 to >120 ng/dL.) during hospitalization.
Three infants died; two had stage III NEC, and one had congenital cytomegalovirus infection. Two additional infants had stage II–III NEC but survived. One infant had grade 3 IVH, and five had laser surgery for ROP. Three infants had a diagnosis of moderate BPD (Supplementary Table 1).
Discussion
Our study found that 18% of VLBW infants had biochemical evidence of VDD at birth; 40% of infants born at ≤28 weeks of gestation, and 3% of infants born between 28 and 35 weeks of gestation. Previous studies had also reported that extremely preterm infants had higher risk for low vitamin D levels. Burris et al. and Joung et al. reported the prevalence of VDD 18% in infants <29 weeks and 14.4% in those <32 weeks in the Boston area [8, 9], while Fort et al. reported VDD in 67% of infants born at 23–27 weeks in Birmingham [10]. McCarthy et al. found 80% of infants born <32 weeks with VDD in Dublin, Ireland [20]. Fetus depends entirely on their mother for vitamin D supply, and most of the transfer occurs during the third trimester. Cord serum 25(OH)D concentrations are about 67–87% of maternal 25(OH)D concentrations [2]. Thus, premature infants, especially those born before the third trimester, had high risk of VDD at birth. Several surveys have shown a high rate of low vitamin D levels in pregnant women globally, particularly in those with lower dietary intake of vitamin D or limited sunshine exposure [12,13,14]. Our study was conducted in the inner city of Chicago, which has long winters, and the majority of mothers were African American. However, we did not find significant association of low vitamin D levels with race or season of birth. This is in contrast to other studies [8,9,10], which found higher prevalence of VDD in infants born in winter and delivered to African American mothers. In our study, the majority (89%) of mothers reported having taken prenatal vitamins during pregnancy. This may also explain the lower prevalence of VDD in more mature infants at birth. SGA had a lower risk of VDD and VDI compared to AGA infants owing to higher GA at birth, and consequently longer duration of maternal placental transfer of vitamin D.
Most ELBW infants are dependent on parenteral nutrition for their vitamin D supply in the first few weeks of life because of delay in starting and advancing due to feeding problems, NEC, and other conditions that preclude enteral feeding; or being fed with unfortified breast milk, which contains negligible amount of vitamin D. There are very few studies or recommendations available for vitamin D supplementation in parenteral nutrition [22]. In our study, most infants weighing <1000 g received vitamin D almost exclusively from parenteral nutrition during the first 2 weeks of life, while most infants with weights between 1000 and 1500 g received their vitamin D mainly from parenteral nutrition in the first 1 week. VDD and VDI were corrected within the first 2 weeks of life while the infants received parenteral nutrition. The dose of vitamin D in parenteral nutrition was sufficient to normalize 25(OH) D levels in our study population. The manufacturer’s formulation is currently stratified based on weight, which is 120 IU/day for infants weighing <1000 g and 260 IU/day for infants weighing 1000–3000 g. This finding has not been verified by any previous study in the neonatal population to the best of our knowledge.
Our finding also supports the AAP’s recommendation of oral vitamin D intake of 200–400 IU/day for VLBW infants. In our study, all the VLBW infants who had VDD at birth achieved and maintained VDS regardless of the route that vitamin D was given. In the United States, fortified breast milk or premature formula contains enough vitamin D for growing premature infants. Of note, unfortified breast milk, elemental or semi-elemental formulas, transitional formula, and regular infant formula contain far less amount of vitamin D as needed for premature infants, additional supplementation is advised [23,24,25]. Our study found that VLBW infants who received daily vitamin D >600 IU had a higher risk of VDE. As such, caution must be exercised in prescribing additional vitamin D supplements once the infants start receiving full feeds of fortified human milk or preterm formula. Excessive 25(OH)D levels also occurred in a few infants whose vitamin D intake was ≤400 IU/day. Therefore, it is prudent to check 25(OH)D levels in this population to optimize vitamin D intake.
Our findings are in agreement with those of previous randomized studies [18,19,20]. Koo et al. [18] reported that VLBW infants fed a high-Ca/high-P content preterm formula could maintain normal serum vitamin D concentrations at 28 days with an average vitamin D intake of about 160 IU/day. Backström et al. [19] found that premature infants with gestation age <33 weeks receiving 200–400 IU/kg of vitamin D in the first 6 weeks after birth had sustained normal 25(OH)D concentrations by 12 weeks. In contrast, Fort et al. [11] reported that a daily vitamin D intake (parenteral and feeds) of 200 or 400 IU/day for extremely premature infants only reduced the VDD by day 28, while intake of 1000 IU/day corrected vitamin deficiency [11]. Others reported similar findings to Fort [10, 21,22,23,24,25]. Some of these discrepancies might be explained by differences in types of vitamin D assay or lengths of time between blood sampling and laboratory measurements [31]. The half-life of 25(OH)D3 is approximately 2–3 weeks. We followed 25(OH)D weekly during the first week and biweekly until discharge to track fluctuations in serum levels with varying vitamin D doses. The strength of our study is the systematic collection of vitamin D data. We calculated the total amount of vitamin D intake for each infant based on their parenteral/enteral nutrition and supplementation. Serum samples for 25(OH)D measurements were collected every 1–2 weeks. The assay measured both the vitamin D2 and vitamin D3 metabolites. The analyses were performed the same day as the blood was collected. This allowed us to investigate the efficacy of vitamin D absorption in a timely fashion. More studies are needed to clarify the variations among different reports.
The absorption of vitamin D by different populations from various sources might not be the same. In our study, all 15 VLBW infants who had VDD at birth received vitamin D 120–400 IU/day from parenteral nutrition and feeds were able to correct VDD in 1–2 weeks. Infants from other populations or ones who received enteral feedings with lower vitamin D content might require higher vitamin D intake.
The safe upper levels of 25(OH)D have been designated as a level of 100 ng/mL. Infants with measurements above 100 ng/mL are considered to have VDE and are at risk for vitamin D intoxication [15]. Cases of reported vitamin D intoxication presented with severe hypercalcemia, hypercalciuria, and nephrocalcinosis. In our study, we did not detect hypercalcemia in any infants when VDE was detected. Renal ultrasound to detect renal stone was not routinely performed. In light of this limitation, we might have missed some cases of nephrocalcinosis related to hypervitaminosis D.
Healthy bone mineralization depends on adequate total calcium and phosphorus absorption. Vitamin D enhances the absorption of calcium and phosphorus from the intestine. In our study, normal or high vitamin D concentrations did not prevent VLBW infants from developing hypophosphatemia or osteopenia of prematurity if the calcium and phosphorus intake was low. A previous study showed that additional vitamin D supplementation to premature infants fed with premature formula did not increase enteral calcium absorption [32]. Therefore, it is important that once premature infants achieve VDS, strategies should be established to ensure adequate mineral intake. Differences in preterm infant formulas between the United States and European nations may account for differences in vitamin D intake guidelines between the AAP and ESPGHAN.
In our study, VLBW infants who had VDD/VDI at birth were more likely to develop RDS and NEC. Low GA is the leading cause of RDS and NEC. Whether VDD/VDI is a contributing factor to these complications is beyond the scope of this study. There are other limitations to our study. We did not measure bone mineral densities for the study infants. We also did not follow the vitamin D status at outpatient clinics in eight infants whose 25(OH)D were >100 ng/mL at discharge. However, we discontinued fortified breast milk or premature formula at discharge, which would reduce their total vitamin D intake. As the daily vitamin D intake decreased, we expected the levels to normalize.
Conclusion
In our study population, VDD and VDI were common in VLBW infants at birth. Vitamin D intake of 120–260 IU from parenteral and 200–400 IU/day from enteral route were sufficient for VLBW infants to correct VDD and VDI by 2–3 weeks of postnatal age and maintain normal levels thereafter. Vitamin D intake higher than 600 IU/day increased the risk of VDE. Our results are also in agreement with the AAP’s recommended oral dose of vitamin D for preterm infants.
References
Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96:365–408.
Taylor SN, Hollis BW, Wagner CL. Vitamin D needs of preterm infants. NeoReviews. 2009;10:e590–e599.
Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M.Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398–417.
Dinlen N, Zenciroglu A, Beken S, Dursun A, Dilli D, Okumus N. Association of vitamin D deficiency with acute lower respiratory tract infections in newborns. J Matern Fetal Neonatal Med. 2016;29:928–32.
Grant CC, Kaur S, Waymouth E, Mitchell EA, Scragg R, Ekeroma A, et al. Reduced primary care respiratory infection visits following pregnancy and infancy vitamin D supplementation: randomised controlled trial. Acta Paediatr. 2015;104:396–404.
Maxwell CS, Carbone ET, Wood RJ. Better newborn vitamin D status lowers RSV-associated bronchiolitis in infants. Nutr Rev. 2012;70:548–52.
Mandell E, Seedorf G, Gien J, Abman SH. Vitamin D treatment improves survival and infant lung structure after intra-amniotic endotoxin exposure in rats: potential role for the prevention of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2014;306:L420–L428.
Joung KE, Burris HH, Marter LJV, McElrath TF, Michael Z, Tabatabai P, et al. Vitamin D and bronchopulmonary dysplasia in preterm infants. J Perinatol. 2016;36:878–82.
Burris HH, Van Marter LJ, McElrath TF, Tabatabai P, Litonjua AA, Weiss ST, et al. Vitamin D status among preterm and full-term infants at birth. Pediatr Res. 2014;75:75–80.
Monangi N, Slaughter JL, Dawodu A, Smith C, Akinbi HT. Vitamin D status of early preterm infants and the effects of vitamin D intake during hospital stay. Arch Dis Child Fetal Neonatal Ed. 2014;99:F166–F168.
Fort P, Salas AA, Nicola T, Craig CM, Carlo WA, Ambalavanan N. A comparison of three vitamin D dosing regimens in extremely preterm infants: a randomized controlled trial. J Pediat.r 2016;174:132–8.e1.
Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr. 2007;137:447–52.
Cadario F, Savastio S, Pozzi E, Capelli A, Dondi E, Gatto M, et al. Vitamin D status in cord blood and newborns: ethnic differences. Ital J Pediatr. 2013;39:35.
Burris HH, Rifas-Shiman SL, Camargo CA Jr, Litonjua AA, Huh SY, Rich-Edwards JW, et al. Plasma 25-hydroxyvitamin D during pregnancy and small-for-gestational age in black and white infants. Ann Epidemiol. 2012;22:581–6.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30.
Abrams SA.Committee on Nutrition Calcium and vitamin D requirements of enterally fed preterm infants. Pediatrics. 2013;131:1676–83.
Agostoni C, Buonocore G, Carnielli VP, Darmaun D, De Curtis M, Decsi T, et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Paediatr Gastroenterol Nutr. 2010;50:85–91.
Koo WWK, Krug-Wispe S, Neylan M, Succop P, Oestreich AE, Tsang RC. Effect of three levels of vitamin D intake in preterm infants receiving high mineral-containing milk. J Pediatr Gastroenterol Nutr. 1995;21:182–89.
Backström MC, Mäki R, Kuusela AL, Sievanen H, Koivisto A, Ikonen R, et al. Randomised controlled trial of vitamin D supplementation on bone density and biochemical indices in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1999;80:F161–F166.
McCarthy RA, McKenna MJ, Oyefeso O, Uduma O, Murray BF, Brady JJ, et al. Vitamin D nutritional status in preterm infants and response to supplementation. Br J Nutr. 2013;110:156–63.
Anderson-Berry A, Thoene M, Wagner J, Lyden E, Jones G, Kaufmann M, et al. Randomized trial of two doses of vitamin D3 in preterm infants <32 weeks: Dose impact on achieving desired serum 25(OH)D3 in a NICU population. PLoS One. 2017;12:e0185950 https://doi.org/10.1371/journal.pone.0185950.
Nehra D, Carlson SJ, Fallon EM, Kalish B, Potemkin AK, Gura KM, et al. A.S.P.E.N. clinical guidelines: nutrition support of neonatal patients at risk for metabolic bone disease. J Parenter Enter Nutr. 2013;37:570–98. SepPMID: 23685349.
Premature Infant Formulas and Products. Pediatric Product Guide (p. 97–128). 2018 Mead Johnson & Company LLC. HCP Mead Johnson. (n.d.). https://www.hcp.meadjohnson.com/s/products?category=Formula. Accessed 26 Aug 2021.
Streym SV, Højskov CS, Møller UK, Heickendorff L, Vestergaard P, Mosekilde L, et al. Vitamin D content in human breast milk: a 9-mo follow-up study. Am J Clin Nutr. 2016;103:107–14.
Dror DK, Allen LH. Overview of nutrients in human milk. Adv Nutr. 2018;9:278S–294.
Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59.
Kliegman RM, Fanaroff AA. Necrotizing enterocolitis. N Engl J Med. 1984;310:1093–103.
Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–94.
Volpe JJ, Inder TE, Darras BT, de Vries LS, du Plessis AJ, Neil JJ, et al. Volpe’s neurology of the newborn. 6th ed. Philadelphia: Elsevier; 2017.
Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723.
Vogiatzi MG, Jacobson-Dickman E, DeBoer MD.Drugs, and Therapeutics Committee of the Pediatric Endocrine Society Vitamin D supplementation and risk of toxicity in pediatrics: a review of current literature. J Clin Endocrinol Metab. 2014;99:1132–41.
Natarajan CK, Sankar MJ, Agarwal R, Pratap OT, Jain V, Gupta N, et al. Trial of daily vitamin D supplementation in preterm infants. Pediatrics. 2014;133:e628–e634.
Author information
Authors and Affiliations
Contributions
MA conceptualized and designed the study and data collection instruments. He collected data, carried out the initial analyses, and drafted, revised the initial manuscript. S-YW conceptualized and designed the study, coordinated and supervised data collection, drafted the initial manuscript, reviewed and revised the manuscript. MK contributed to the statistical plan and analyzed the data with MA. VD designed the study and critically reviewed and revised the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Adnan, M., Wu, SY., Khilfeh, M. et al. Vitamin D status in very low birth weight infants and response to vitamin D intake during their NICU stays: a prospective cohort study. J Perinatol 42, 209–216 (2022). https://doi.org/10.1038/s41372-021-01238-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-021-01238-9