Abstract
Background
Nasal continuous positive airway pressure (NCPAP) and high flow nasal cannula (HFNC) are modes of non-invasive respiratory support commonly used after extubation in extremely preterm infants. However, the cardiorespiratory physiology of these infants on each mode is unknown.
Methods
Prospective, randomized crossover study in infants with birth weight ≤1250 g undergoing their first extubation attempt. NCPAP and HFNC were applied randomly for 45 min each, while ribcage and abdominal movements, electrocardiogram, oxygen saturation, and fraction of inspired oxygen (FiO2) were recorded. Respiratory signals were analyzed using an automated method, and differences between NCPAP and HFNC features and changes in FiO2 were analyzed.
Results
A total of 30 infants with median [interquartile range] gestational age of 27 weeks [25.7, 27.9] and birth weight of 930 g [780, 1090] were studied. Infants were extubated at 5 days [2, 13] of life with 973 g [880, 1170] and three failed (10%). No differences in cardiorespiratory behavior were noted, except for longer respiratory pauses (9.2 s [5.0, 11.5] vs. 7.3 s [4.6, 9.3]; p = 0.04) and higher FiO2 levels (p = 0.02) during HFNC compared to NCPAP.
Conclusions
In extremely preterm infants studied shortly after extubation, the use of HFNC was associated with longer respiratory pauses and higher FiO2 requirements.
Similar content being viewed by others
Introduction
In order to improve rates of successful extubation in extremely preterm infants, some form of non-invasive respiratory support must be provided after disconnection from the ventilator.1 Nasal continuous positive airway pressure (NCPAP) is the most commonly used support2 since it promotes upper airway splinting, improves functional residual capacity and thoraco-abdominal synchrony, and decreases work of breathing (WOB).3,4
Over the past years, heated and humidified high flow nasal cannula (HFNC) therapy has emerged as another mode of non-invasive respiratory support in preterm infants.2 Although some degree of positive distending pressure can be generated, its main mechanism of action is through washout of the nasopharyngeal dead space.5,6 A few studies comparing NCPAP and HFNC after extubation found little to no differences in lung mechanics and breathing patterns (PATT).7,8,9,10 However, these infants were older and were studied a few days after extubation. Therefore, these findings cannot be extrapolated to extremely preterm infants during the critical period shortly after extubation. Furthermore, analysis of breathing PATTs was performed manually and in a selected number of breaths. Indeed, clinical evidence of HFNC safety and efficacy in this population is lacking, as a small number of extremely preterm infants were included in randomized trials comparing NCPAP and HFNC after extubation.6,11 Thus, a better understanding of the physiological effects of these modes of support following extubation is necessary in this population.
In recent years, we have developed automated tools to objectively analyze cardiorespiratory signals and define breathing PATTs in preterm infants.12,13,14 Thus, in this randomized crossover study, we used these tools to describe and compare the cardiorespiratory behavior of extremely preterm infants while receiving NCPAP and HFNC shortly after extubation. Given the differences in mechanisms of action of both modes, we hypothesized that a higher incidence of respiratory pauses (PAUs), asynchronous breathing (ASB), and bradycardias (BDYs) will be observed during HFNC therapy.
Methods
Study design and participants
A prospective, unblinded, randomized crossover study was performed in the neonatal intensive care units at the Royal Victoria Hospital and Montreal Children’s Hospital (McGill University Health Center, Montreal, Canada) from October 2013 to August 2015. Inclusion criteria were intubated infants with a birth weight (BW) ≤1250 g undergoing their first elective extubation. Infants with major congenital anomalies, neuromuscular disease, or clinical instability in the immediate period following extubation were excluded. The institutional review board at each institution approved the study, and written parental consent was obtained. The trial was registered at clinicaltrials.gov (NCT03649282), and randomization was performed prior to extubation using a computer-generated blocked randomization sequence.
Clinical decisions pertaining to weaning from mechanical ventilation, time of extubation, and type of respiratory support used after extubation (aside from the study recording period) were made by the medical team. Also, all infants received caffeine 24 h prior to extubation.
Instrumentation and recordings
Prior to extubation, three electrocardiogram (ECG) electrodes were placed on the infant’s chest and limbs at least 1 cm apart from the existing leads to prevent interference (Vermed, Buffalo, NY, USA, © 2010). Respiratory movements were measured using two respiratory inductance plethysmography (RIP) bands: around the infant’s ribcage (RCG) at the level of the nipple line, and around the abdomen (ABD) 0.5 cm above the umbilicus (Viasys® Healthcare, Conshohocken, PA, USA). Oxygen saturation (SpO2) was recorded using a pulse oximeter (Masimo Radical®, Irvine, CA, USA).
Following extubation, a short period of approximately 30 min was allowed before the infants were switched to the mode determined by the randomization (NCPAP or HFNC) and recordings were initiated. Infants were kept in a supine position and received 45 min of each mode. NCPAP was applied at a pressure level of 5–6 cmH2O, delivered either by the bubble CPAP system or by the ventilator (Babylog 8000 or VN500, Dräger Medical Inc., Telford, PA, USA). As per standard of care, all efforts were made to minimize leaks by using the appropriate size of bi-nasal prongs (Hudson RCI, Wayne, PA, USA), applying the Cannulaide® (Salter Labs, Lake Forest, IL, USA), and using a chinstrap. HFNC therapy was delivered using the Optiflow system (Fisher & Paykel®, Auckland, New Zealand) at flow rates of 5–6 L/min using a cannula/nostril diameter ratio between 0.5 and 0.8.
During recordings, the fraction of inspired oxygen (FiO2), NCPAP pressure, or HFNC flow rate could be adjusted if necessary. The SpO2 target range was between 88 and 92%, and any adjustment was recorded. The study could be interrupted if the infant exhibited significant episodes of desaturations (DSTs) and/or PAUs refractory to these adjustments, in which case the infant would be excluded. At the end, infants were placed on the type of non-invasive support decided a priori by the medical team.
Cardiorespiratory signal acquisition
Cardiorespiratory signals (RCG, ABD, PPG, and SpO2) were continuously acquired using the PowerLab 16/30 analog-digital data acquisition system (ADInstruments, Australia, © 2009). Signals were anti-alias filtered at 500 Hz and sampled at 1000 Hz with a 16-bit analog-to-digital resolution. ECG was recorded using an FE132 bioamplifier (ADInstruments, Australia, © 2009) connected to the PowerLab. All signals were stored on a research computer. Changes in FiO2 and any interventions were recorded in real time as text comments in the LabChart software (ADInstruments, Australia, © 2009). Each LabChart file was then converted to MATLABTM format for subsequent signal analysis (The MathWorks Inc., Natick, MA, USA).
Clinical data
The following clinical data were prospectively collected: baseline demographics (gestational age, sex, Apgar scores, weight, day of life at extubation, and postmenstrual age at extubation), pre-extubation blood gases (pH, PCO2, bicarbonate, and base excess), and use of postnatal steroids prior to extubation. Non-invasive respiratory support settings were recorded at the beginning and throughout the study when a change from baseline occurred (NCPAP pressure, HFNC flow rate, FiO2). Extubation failure was defined as the need for reintubation within 7 days of extubation, and the primary reason for reintubation was recorded.
Signal analysis
Respiratory signals
The first 15 min on each mode were considered a transition period and not included in the analysis. Respiratory signals were processed using an Automated Unsupervised Respiratory Event Analysis system (AUREA); further details are described elsewhere.12 AUREA computes a series of cardiorespiratory metrics related to respiratory frequency, thoraco-abdominal synchrony, and movement artifact (MVT) at every time point in a sample-by-sample manner at a sampling rate of 50 Hz. The metrics are then used by a series of k-means classifiers to objectively classify the data sample at each time point into one of four classes, or PATTs:
- (a)
PAU: >2 s cessation of breathing evidenced by minimal RCG and ABD movement.
- (b)
ASB: >2 s during which RCG and ABD are out of phase. By AUREA’s classification, the phase angle that split synchrony and asynchrony in this population was 83°.
- (c)
Synchronous breathing (SYB): >2 s during which RCG and ABD are in phase.
- (d)
MVT: >2 s during which RCG and ABD display artifacts associated with body movements or nurse handling. During MVT, RCG, and ABD are corrupted by larger amplitude and low-frequency motions not related to respiration.
Heart rate
The heart rate (HR) at each time point was estimated using the PPG to identify periods of BDY, defined as HR <100 beats/min for at least 2 consecutive seconds during artifact-free periods. Artifact was detected using a PPG MVT detector described fully elsewhere.15
Oxygenation (SpO2 and FiO2)
The SpO2 signal was analyzed for episodes of DST, defined as a period of >20 s without MVT during which the SpO2 was <85%. Baseline FiO2 range and FiO2 changes were computed for every patient during both modes of support.
Cardiorespiratory events
We also defined cardiorespiratory events that would raise clinical concerns. Three types of cardiorespiratory events were defined and analyzed:
- (a)
Apnea (APN): PAU duration >20 s.
- (b)
PAU with DST (PAU + DST): PAU with a duration of 5–20 s + SpO2 < 85%, starting anywhere from the onset of PAU to within 20 s from the end of PAU.
- (c)
PAU with BDY (PAU + BDY): PAU with a duration of 5–20 s + HR < 1 standard deviation from the mean, starting anywhere from the onset of PAU to within 20 s from the end of PAU. The objective of this definition was to include situations where a significant decrease in HR occurred during a respiratory event despite the HR ≥100 beats/min.
Cardiorespiratory behavior analysis
For analysis of the cardiorespiratory behavior during NCPAP and HFNC, a total of 9 PATTs were included: PAU, SYB, ASB, and MVT (from the RIP signals); BDY (from the PPG signal); DST (from the SpO2 signal); and the cardiorespiratory events APN, PAU + DST, and PAU + BDY. For each of these 9 PATTs, the following features were extracted:
- (a)
Length of each occurrence: TPATT (s).
- (b)
Total duration during the entire recording: TPATTTOT (s).
- (c)
Total number of occurrences: NPATTTOT (#).
- (d)
Frequency of occurrence: The total number of occurrences divided by the duration of the recording: FPATT = (60*NPATTTOT)/TTOT (#/min).
- (e)
Density of occurrence: The total duration of a pattern divided by the duration of the recording: DPATT = TPATTTOT/TTOT (unitless).
- (f)
Length of the longest occurrence: TPATTMAX (s).
- (g)
Paired differences between HFNC and NCPAP for each of the features (a)–(f). For example, the paired difference for the length of the longest PAU occurrence is:
Changes in FiO2 and cardiorespiratory behavior for periods of matched FiO2 during NCPAP and HFNC were also analyzed, to explore if changes in FiO2 could affect cardiorespiratory behavior.
Sample size and statistical analysis
A convenience sample size of 30 infants was chosen as it could reasonably be achieved in a 2-year period. Descriptive data was presented as median and interquartile range (IQR) for continuous variables or count (%) for categorical variables. Features were compared using a paired analysis, whereby each patient is his/her own control, thereby accounting for intra-subject variability and providing greater comparative strength than an unpaired analysis. Since the features were not normally distributed, the Wilcoxon’s rank-sum and signed-rank nonparametric tests were used for all paired comparisons; p values <0.05 were considered significant.
Results
Study population and clinical data
A total of 152 eligible infants were admitted to the participating centers during the study period and 30 were included. Details concerning patient recruitment are provided in Fig. 1. Included infants had a median gestational age of 27 weeks [25.7, 27.9], BW of 930 g [780, 1090], extubated at a median age of 5 days of life [2, 13], and 3 (10%) were reintubated within 7 days of extubation. Table 1 provides details of the population demographics. Fourteen infants (47%) were studied on NCPAP first and 16 (53%) on HFNC first. During recordings, 3 (10%) infants required tactile stimulation while on HFNC due to BDYs detected on the unit monitor, whereas no infant required stimulation during NCPAP. No changes in NCPAP pressure or HFNC flow rate were made during any of the recordings.
Cardiorespiratory behavior
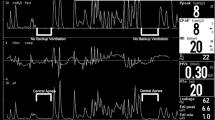
A total of 105,383 breaths were analyzed: 55,015 breaths during NCPAP and 50,368 breaths during HFNC. Cardiorespiratory signals and resulting PATTs (Fig. 2) were analyzed for all 30 infants during NCPAP and HFNC. The properties of 6 PATTs features are detailed in Table 2.
Cardiorespiratory signals and the pattern signal classified by Automated Unsupervised Respiratory Event Analysis system (AUREA) during nasal continuous positive airway pressure (NCPAP) and high flow nasal cannula (HFNC). Legend: Behavior under NCPAP (upper panel) and HFNC (lower panel). This example shows longer maximum pause durations during HFNC. The signals acquired are: (a) electrocardiogram (ECG); (b) ribcage movements (RCG) using RIP; (c) abdomen movements (ABD) using RIP; (d) photoplethysmography (PPG); (e) oxygen saturation (SpO2). The RIP signals acquired in b, c) produced the sample-by-sample pattern signal shown in f
PAUs: There were no differences for most of the analysis of respiratory pauses between NCPAP and HFNC, except for the difference in the maximum pause duration \(\left( {\Delta {T}^{{\mathrm{PAU}}}_{{\mathrm{MAX}}}} \right)\), which was significantly longer during HFNC (p = 0.04), as shown in Fig. 3. This difference was not affected by which therapy was applied initially as TPAUMAX was longer under HFNC in 10 (33%) patients initially studied on NCPAP and 11 (37%) who initially received HFNC.
Longest pauses (TPAUMAX) observed during high flow nasal cannula (HFNC) and nasal continuous positive airway pressure (NCPAP). Legend: Boxplot of differences in the longest pauses \(\left( {\Delta {T}^{{\mathrm{PAU}}}_{{\mathrm{MAX}}}} \right)\) of all patients. The longest occurring pauses were significantly higher while receiving HFNC when compared to NCPAP
SYB, ASB, and MVT: All patients experienced periods of SYB, ASB, and MVT under both modes of non-invasive respiratory support. No significant differences in these PATTs were noted.
Oxygenation: No significant differences in episodes of DST were observed between the two modes of support. Episodes of DST >20 s occurred at least once in 15 patients (50%) during NCPAP and 20 patients (67%) during HFNC (p = 0.3). The median length of an episode was 34.9 s [25.3, 62.0] during NCPAP and 32.2 s [25.3, 51.2] during HFNC. Importantly, 75% (15/20) of those infants who had a DST episode under HFNC also had an episode under NCPAP.
The minimum FiO2 required during both supports was 21% (p = 0.05) and the median FiO2 range (i.e., highest needs minus lowest needs) was 2% [0, 6] under NCPAP and 5% [0, 10] under HFNC (p = 0.09). A significant difference was noted for the lowest and highest FiO2 levels between HFNC and NCPAP (Fig. 4).
Fraction of inspired oxygen (FiO2) during high flow nasal cannula (HFNC) and nasal continuous positive airway pressure (NCPAP). Legend: Boxplots of the paired difference for the lowest and highest FiO2 requirements during HFNC and NCPAP (using each infant as their own control). A positive value on either boxplot indicates that a patient required a higher FiO2 value while receiving HFNC than under NCPAP. As indicated by the positive values for both boxplots, a higher FiO2 requirement was required during HFNC therapy
HR: No significant differences in episodes of BDY were observed. Indeed, only a few episodes of BDY >2 s were identified: four patients (13%) during NCPAP and five patients (17%) during HFNC (p = 0.8). Of the infants who had a BDY episode during HFNC, 40% (2/5) also had a BDY episode during NCPAP. The median TBDY during NCPAP was 6.4 s [3.4, 12.2] and during HFNC was 5.7 s [3.2, 17.2].
Cardiorespiratory events: There were no significant differences between the two modes of support for the three cardiorespiratory events analyzed: APN, PAU + DST, and PAU + BDY. Three APN events of 21 s mean duration were detected during HFNC and none during NCPAP.
Cardiorespiratory behavior during matched FiO2: Fourteen (47%) patients had segments of HFNC and NCPAP recordings during which the FiO2 value was identical and no significant differences in cardiorespiratory behavior were noted.
Discussion
In this study, we describe the cardiorespiratory behavior of extremely preterm infants shortly after extubation while receiving NCPAP and HFNC. By using an automated and objective analysis of cardiorespiratory signals several features were evaluated. Interestingly, even though infants were exposed to NCPAP and HFNC for a short period of time, this analysis was able to demonstrate longer durations of respiratory pauses and higher FiO2 requirements during HFNC. Therefore, these findings provide further understanding on the physiological effects of NCPAP and HFNC in extremely preterm infants during the critical period shortly after extubation.
Few studies have investigated the respiratory behavior of preterm infants receiving NCPAP or HFNC following extubation. In a crossover study, Saslow et al.7 evaluated lung mechanics in 18 infants using RIP signals analysis. No differences in WOB were noted between NCPAP and HFNC. More recently, De Jongh et al. studied 20 infants extubated for ≥48 h and on non-invasive respiratory support.16 Using a crossover random design, WOB indices were evaluated from uncalibrated RIP signal recordings of 2 to 4 min during four different modes: NCPAP at 5 and 6cmH2O and HFNC at flow rates of 3 and 5 lpm. Although statistically significant differences on WOB indices were noted between NCPAP and HFNC, they were not clinically meaningful, as suggested by the large overlap of the 95% confidence intervals. Differently from our study, infants were more mature and with higher weights at the time of recordings. Furthermore, analysis of the RIP signals was done in a selected number of breaths (over a shorter time frame) and did not provide information on respiratory pauses. In the present study, by using an automated and objective method, we were able to analyze all breaths. In another trial, Campbell et al.17 studied 40 preterm infants with GA of 27.5 weeks extubated around the second day of life to either NCPAP or HFNC. APN was defined based on nurses’ notes as a pause in breathing for >20 s and BDY as HR <80 bpm for >10 s, over a period of 7 days of treatment. Extubation failure was significantly higher in the HFNC group (60% vs. 15%, p = 0.003) and all infants were reintubated due to severe APN or increased frequency of APNs. The duration of the APN events was not reported. Due to the study protocol, we included a relatively more stable population, as 90% of the infants were successfully extubated. This selection bias could be the reason for the lack of differences in SYB and ASB breaths (WOB) or MVT (agitation and/or distortion) between the modes. Nevertheless, longer respiratory pauses were noted during HFNC therapy. Although small, such differences in respiratory pauses in combination with higher FiO2 requirements may reflect some instability of the cardiorespiratory system during HFNC therapy after only 45 min of exposure and 30 min of analysis. As APNs and increased FiO2 are the most common causes of extubation failure,18 our findings raise concerns about the widespread use of HFNC therapy in this immature population as a mode of post-extubation support.
Campbell et al.17 have also investigated for differences in FiO2 requirements between NCPAP and HFNC. Changes were calculated as the difference between the FiO2 over the 7 days post extubation and up to 12 h pre-extubation; a significantly higher FiO2 change (4.7% vs. 1.0%; p = 0.025) was observed in infants receiving HFNC. These results are similar to our findings, despite the fact that we studied patients during a much shorter period of exposure to both modes.
The present study has some limitations. Recordings and changes of respiratory therapies shortly after extubation are difficult to perform due to patient instability, which limited our protocol to 45 min. We recognize that longer periods would provide better evaluations of cardiorespiratory behavior. Nonetheless, the study has several strengths. We performed a randomized crossover trial where each infant was exposed to both modes of support in a random order. Signals were analyzed using a unique, automated, unsupervised, and objective system with the ability to perform timely classification of breathing PATTs on a sample-by-sample basis for over 100,000 breaths, even in high noise conditions. This new approach allowed computation of a large number of features to describe cardiorespiratory behavior in 30 infants.
In summary, extremely preterm infants studied shortly after extubation exhibited significantly longer respiratory pauses and higher FiO2 requirements while receiving HFNC when compared to NCPAP. These findings raise concerns on the use of HFNC after extubation in this population and future studies, including a larger number of infants are necessary.
References
Ferguson, K. N., Roberts, C. T., Manley, B. J. & Davis, P. G. Interventions to improve rates of successful extubation in preterm infants: a systematic review and meta-analysis. JAMA Pediatr. 171, 165–174 (2017).
Al-Mandari, H. et al. International survey on periextubation practices in extremely preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 100, F428–F431 (2015).
Gupta, S. & Donn, S. M. Continuous positive airway pressure: physiology and comparison of devices. Semin. Fetal Neonatal Med. 21, 204–211 (2016).
Dysart, K. C. Physiologic basis for nasal continuous positive airway pressure, heated and humidified high-flow nasal cannula, and nasal ventilation. Clin. Perinatol. 43, 621–631 (2016).
Manley, B. J. & Owen, L. S. High-flow nasal cannula: mechanisms, evidence and recommendations. Semin. Fetal Neonatal Med. 21, 139–145 (2016).
Wilkinson, D., Andersen, C., O’Donnell, C. P., De Paoli, A. G. & Manley, B. J. High flow nasal cannula for respiratory support in preterm infants. Cochrane Database Syst. Rev. 2, CD006405 (2016).
Saslow, J. G. et al. Work of breathing using high-flow nasal cannula in preterm infants. J. Perinatol. 26, 476–480 (2006).
Shetty, S., Sundaresan, A., Hunt, K., Desai, P. & Greenough, A. Changes in the use of humidified high flow nasal cannula oxygen. Arch. Dis. Child. Fetal Neonatal Ed. 101, F371–F372 (2016).
Lavizzari, A. et al. Respiratory mechanics during NCPAP and HHHFNC at equal distending pressures. Arch. Dis. Child. Fetal neonatal Ed. 99, F315–F320 (2014).
Sreenan, C., Lemke, R. P., Hudson-Mason, A. & Osiovich, H. High-flow nasal cannulae in the management of apnea of prematurity: a comparison with conventional nasal continuous positive airway pressure. Pediatrics 107, 1081–1083 (2001).
Manley, B. J. Nasal high-flow therapy for preterm infants. Clin. Perinatol. 43, 673–691 (2016).
Robles-Rubio, C. A., Brown, K. A. & Kearney, R. E. Automated unsupervised respiratory event analysis. Conf Proc IEEE Eng Med Biol Soc. 2011, 3201–3204 (2011).
Precup, D. et al. Prediction of extubation readiness in extreme preterm infants based on measures of cardiorespiratory variability. Conf Proc IEEE Eng Med Biol Soc. 2012, 5630–5633 (2012).
Robles-Rubio, C. A. et al. Automated analysis of respiratory behavior in extremely preterm infants and extubation readiness. Pediatr Pulmonol. 50, 479–486 (2015).
Robles-Rubio, C. A., Brown, K. A. & Kearney, R. E. A new movement artifact detector for photoplethysmographic signals. Conf Proc IEEE Eng Med Biol Soc. 2013, 2295–2299 (2013).
de Jongh, B. E. et al. Work of breathing indices in infants with respiratory insufficiency receiving high-flow nasal cannula and nasal continuous positive airway pressure. J. Perinatol. 34, 27–32 (2014).
Campbell, D. M., Shah, P. S., Shah, V. & Kelly, E. N. Nasal continuous positive airway pressure from high flow cannula versus infant flow for preterm infants. J. Perinatol. 26, 546–549 (2006).
Manley, B. J., Doyle, L. W., Owen, L. S. & Davis, P. G. Extubating extremely preterm infants: predictors of success and outcomes following failure. J. Pediatr. 173, 45–49 (2016).
Acknowledgements
We are indebted to the babies and their families for their consent to participate in this study. L.J.K., R.E.K., K.A.B., and G.S.A. were funded by the Canadian Institutes of Health Research. L.J.K. was also funded by the Natural Sciences and Engineering Research Council of Canada. K.A.B. was also funded by the Queen Elizabeth Hospital of Montreal Foundation Chair in Pediatric Anesthesia. The funding bodies had no role in the design, collection, analysis, or interpretation of the data.
Author information
Authors and Affiliations
Contributions
L.J.K. contributed to the development of the protocol and study design, assisted in acquiring the recordings, analyzed the data, and wrote the manuscript. W.S. participated in the development of the protocol and study design, and was responsible for patient screening, enrollment, data collection, and recordings. S.L. assisted in patient screening, enrollment, data collection, and recordings. S.R. assisted in patient screening, enrollment, data collection, and recordings. K.A.B. participated in the development of the protocol, and provided critical input into study design, data analyses, and writing of the manuscript. R.E.K. participated in the development of the protocol, and provided critical input into study design, data analyses, and writing of the manuscript. G.M.S. supervised the design and execution of the study, and provided critical input into the final data analyses, and writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kanbar, L.J., Shalish, W., Latremouille, S. et al. Cardiorespiratory behavior of preterm infants receiving continuous positive airway pressure and high flow nasal cannula post extubation: randomized crossover study. Pediatr Res 87, 62–68 (2020). https://doi.org/10.1038/s41390-019-0494-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0494-5
This article is cited by
-
Automated prediction of extubation success in extremely preterm infants: the APEX multicenter study
Pediatric Research (2023)
-
Adjustment of high flow nasal cannula rates using real-time work of breathing indices in premature infants with respiratory insufficiency
Journal of Perinatology (2021)
-
Continuous positive airway pressure and high flow nasal cannula: the search for effectiveness continues
Pediatric Research (2020)