Abstract
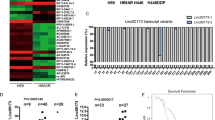
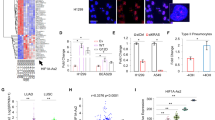
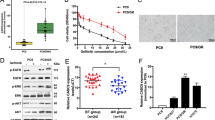
The focal adhesion kinase (FAK) tyrosine kinase is activated and upregulated in multiple cancer types including small cell lung cancer (SCLC). However, FAK inhibitors have shown limited efficacy in clinical trials for cancer treatment. With the aim of identifying potential therapeutic strategies to inhibit FAK for cancer treatment, we investigated long non-coding RNAs (lncRNAs) that potentially regulate FAK in SCLC. In this study, we identified a long non-coding RNA LINC01089 that binds and inhibits FAK phosphorylation (activation). Expression analysis revealed that LINC01089 was downregulated in SCLC tissues and negatively correlated with chemoresistance and survival in SCLC patients. Functionally, LINC01089 inhibited chemoresistance and progression of SCLC in vitro and in vivo. Mechanistically, LINC01089 inhibits FAK activation by blocking binding with Src and talin kinases, while FAK negatively regulates LINC01089 transcription by activating the ERK signaling pathway to recruit the REST transcription factor. Furthermore, LINC01089-FAK axis mediates the expression of drug resist-related genes by modulating YBX1 phosphorylation, leading to drug resistance in SCLC. Intriguingly, the FAK-LINC01089 interaction depends on the co-occurrence of the novel FAK variant and the non-conserved region of LINC01089 in primates. In Conclusion, our results indicated that LINC01089 may serve as a novel high-efficiency FAK inhibitor and the FAK-LINC01089 axis represents a valuable prognostic biomarker and potential therapeutic target in SCLC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The RIP-RNA sequencing data was available in the Sequence Read Archive (SRA) with the accession number PRJNA1002414.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7–33.
Rudin CM, Brambilla E, Faivre-Finn C, Sage J. Small-cell lung cancer. Nat Rev Dis Primers. 2021;7:3.
Zugazagoitia J, Paz-Ares L. Extensive-Stage Small-Cell Lung Cancer: First-Line and Second-Line Treatment Options. J Clin Oncol. 2022;40:671–80.
Iams WT, Porter J, Horn L. Immunotherapeutic approaches for small-cell lung cancer. Nat Rev Clin Oncol. 2020;17:300–12.
Hou W, Zhou X, Yi C, Zhu H. Immune Check Point Inhibitors and Immune-Related Adverse Events in Small Cell Lung Cancer. Front Oncol. 2021;11:604227.
Yang S, Zhang Z, Wang Q. Emerging therapies for small cell lung cancer. J Hematol Oncol. 2019;12:47.
Cooper J, Giancotti FG. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell. 2019;35:347–67.
Fu Y, Zhang Y, Lei Z, Liu T, Cai T, Wang A, et al. Abnormally activated OPN/integrin αVβ3/FAK signalling is responsible for EGFR-TKI resistance in EGFR mutant non-small-cell lung cancer. J Hematol Oncol. 2020;13:169.
Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer. 2014;14:598–610.
Tapial Martínez P, López Navajas P, Lietha D. FAK Structure and Regulation by Membrane Interactions and Force in Focal Adhesions. Biomolecules. 2020;10:179.
Hall JE, Fu W, Schaller MD. Focal adhesion kinase: exploring Fak structure to gain insight into function. Int Rev Cell Mol Biol. 2011;288:185–225.
Lietha D, Cai X, Ceccarelli DF, Li Y, Schaller MD, Eck MJ. Structural basis for the autoinhibition of focal adhesion kinase. Cell. 2007;129:1177–87.
Aboubakar Nana F, Vanderputten M, Ocak S. Role of Focal Adhesion Kinase in Small-Cell Lung Cancer and Its Potential as a Therapeutic Target. Cancers (Basel). 2019;11:1683.
Aboubakar Nana F, Hoton D, Ambroise J, Lecocq M, Vanderputten M, Sibille Y, et al. Increased Expression and Activation of FAK in Small-Cell Lung Cancer Compared to Non-Small-Cell Lung Cancer. Cancers (Basel). 2019;11:1526.
Aboubakar Nana F, Lecocq M, Ladjemi MZ, Detry B, Dupasquier S, Feron O, et al. Therapeutic Potential of Focal Adhesion Kinase Inhibition in Small Cell Lung Cancer. Mol Cancer Ther. 2019;18:17–27.
Dawson JC, Serrels A, Stupack DG, Schlaepfer DD, Frame MC. Targeting FAK in anticancer combination therapies. Nat Rev Cancer. 2021;21:313–24.
Quispe PA, Lavecchia MJ, León IE. Focal adhesion kinase inhibitors in the treatment of solid tumors: Preclinical and clinical evidence. Drug Discov Today. 2022;27:664–74.
Statello L, Guo CJ, Chen LL, Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021;22:96–118.
Schmitt AM, Chang HY. Long Noncoding RNAs: At the Intersection of Cancer and Chromatin Biology. Cold Spring Harb Persp Med. 2017;7:a026492.
Slack FJ, Chinnaiyan AM. The Role of Non-coding RNAs in Oncology. Cell. 2019;179:1033–55.
Liu SJ, Dang HX, Lim DA, Feng FY, Maher CA. Long noncoding RNAs in cancer metastasis. Nat Rev Cancer. 2021;21:446–60.
Kumar S, Pandey M, Sharawat SK. Biological functions of long noncoding RNAs and circular RNAs in small-cell lung cancer. Epigenomics. 2020;12:1751–63.
Wang S, Zeng F, Liang S, Wang Q, Wen Y, Wang Q, et al. lncRNA Linc00173 modulates glucosemetabolism and multidrug chemoresistancein SCLC: Potential molecular panel for targeted therapy. Mol Ther. 2021;30:1787.
Niu Y, Ma F, Huang W, Fang S, Li M, Wei T, et al. Long non-coding RNA TUG1 is involved in cell growth and chemoresistance of small cell lung cancer by regulating LIMK2b via EZH2. Mol Cancer. 2017;16:5.
Chen LL. Linking Long Noncoding RNA Localization and Function. Trends Biochem Sci. 2016;41:761–72.
Guo CJ, Ma XK, Xing YH, Zheng CC, Xu YF, Shan L, et al. Distinct Processing of lncRNAs Contributes to Non-conserved Functions in Stem Cells. Cell. 2020;181:621–636.e622.
Zak TJ, Koshman YE, Samarel AM, Robia SL. Regulation of Focal Adhesion Kinase through a Direct Interaction with an Endogenous Inhibitor. Biochemistry. 2017;56:4722–31.
Tsujioka M, Miyazawa K, Ohmuraya M, Nibe Y, Shirokawa T, Hayasaka H, et al. Identification of a novel type of focal adhesion remodelling via FAK/FRNK replacement, and its contribution to cancer progression. Cell Death Dis. 2023;14:256.
Desiniotis A, Kyprianou N. Significance of talin in cancer progression and metastasis. Int Rev Cell Mol Biol. 2011;289:117–47.
Petrich BG. Talin-dependent integrin signalling in vivo. Thrombosis Haemostasis. 2009;101:1020–4.
Frame MC, Patel H, Serrels B, Lietha D, Eck MJ. The FERM domain: organizing the structure and function of FAK. Nat Rev Mol Cell Biol. 2010;11:802–14.
Kuwano M, Shibata T, Watari K, Ono M. Oncogenic Y-box binding protein-1 as an effective therapeutic target in drug-resistant cancer. Cancer Sci. 2019;110:1536–43.
Oda Y, Kohashi K, Yamamoto H, Tamiya S, Kohno K, Kuwano M, et al. Different expression profiles of Y-box-binding protein-1 and multidrug resistance-associated proteins between alveolar and embryonal rhabdomyosarcoma. Cancer Sci. 2008;99:726–32.
Dhillon J, Astanehe A, Lee C, Fotovati A, Hu K, Dunn SE. The expression of activated Y-box binding protein-1 serine 102 mediates trastuzumab resistance in breast cancer cells by increasing CD44+ cells. Oncogene. 2010;29:6294–6300.
Zhang J, Fan JS, Li S, Yang Y, Sun P, Zhu Q, et al. Structural basis of DNA binding to human YB-1 cold shock domain regulated by phosphorylation. Nucleic Acids Res. 2020;48:9361–71.
Prabhu L, Hartley AV, Martin M, Warsame F, Sun E, Lu T. Role of post-translational modification of the Y box binding protein 1 in human cancers. Genes Dis. 2015;2:240–6.
Steiner E, Holzmann K, Elbling L, Micksche M, Berger W. Cellular functions of vaults and their involvement in multidrug resistance. Curr Drug Targets. 2006;7:923–34.
Andrade CBV, Lopes LVA, Ortiga-Carvalho TM, Matthews SG, Bloise E. Infection and disruption of placental multidrug resistance (MDR) transporters: Implications for fetal drug exposure. Toxicol Appl Pharmacol. 2023;459:116344.
Tailor D, Resendez A, Garcia-Marques FJ, Pandrala M, Going CC, Bermudez A, et al. Y box binding protein 1 inhibition as a targeted therapy for ovarian cancer. Cell Chem Biol. 2021;28:1206–1220.e1206.
Sas-Chen A, Aure MR, Leibovich L, Carvalho S, Enuka Y, Körner C, et al. LIMT is a novel metastasis inhibiting lncRNA suppressed by EGF and downregulated in aggressive breast cancer. EMBO Mol Med. 2016;8:1052–64.
Rigiracciolo DC, Cirillo F, Talia M, Muglia L, Gutkind JS, Maggiolini M, et al. Focal Adhesion Kinase Fine Tunes Multifaced Signals toward Breast Cancer Progression. Cancers (Basel). 2021;13:645.
Lai H, Zhao X, Qin Y, Ding Y, Chen R, Li G, et al. FAK-ERK activation in cell/matrix adhesion induced by the loss of apolipoprotein E stimulates the malignant progression of ovarian cancer. J Exp Clin Cancer Res. 2018;37:32.
Tang Y, Jia Z, Xu H, Da LT, Wu Q. Mechanism of REST/NRSF regulation of clustered protocadherin alpha genes. Nucleic Acids Res. 2021;49:4506–21.
Nakano Y, Kelly MC, Rehman AU, Boger ET, Morell RJ, Kelley MW, et al. Defects in the Alternative Splicing-Dependent Regulation of REST Cause Deafness. Cell. 2018;174:536–548.e521.
Paonessa F, Criscuolo S, Sacchetti S, Amoroso D, Scarongella H, Pecoraro Bisogni F, et al. Regulation of neural gene transcription by optogenetic inhibition of the RE1-silencing transcription factor. Proc Natl Acad Sci USA. 2016;113:E91–100.
Hogg SJ, Motorna O, Cluse LA, Johanson TM, Coughlan HD, Raviram R, et al. Targeting histone acetylation dynamics and oncogenic transcription by catalytic P300/CBP inhibition. Mol Cell. 2021;81:2183–2200.e2113.
Wimmers F, Donato M, Kuo A, Ashuach T, Gupta S, Li C, et al. The single-cell epigenomic and transcriptional landscape of immunity to influenza vaccination. Cell. 2021;184:3915–3935.e3921.
Sungalee S, Liu Y, Lambuta RA, Katanayeva N, Donaldson Collier M, Tavernari D, et al. Histone acetylation dynamics modulates chromatin conformation and allele-specific interactions at oncogenic loci. Nat Genet. 2021;53:650–62.
Liu Y, Zhan Y, Chen Z, He A, Li J, Wu H, et al. Directing cellular information flow via CRISPR signal conductors. Nat Methods. 2016;13:938–44.
Zhao G, Gong L, Su D, Jin Y, Guo C, Yue M, et al. Cullin5 deficiency promotes small-cell lung cancer metastasis by stabilizing integrin β1. J Clin Invest. 2019;129:972–87.
Oshita F, Kameda Y, Hamanaka N, Saito H, Yamada K, Noda K, et al. High expression of integrin beta1 and p53 is a greater poor prognostic factor than clinical stage in small-cell lung cancer. Am J Clin Oncol. 2004;27:215–9.
Li N, Zhang JP, Guo S, Min J, Liu LL, Su HC, et al. Down-regulation of β3-integrin inhibits bone metastasis of small cell lung cancer. Mol Biol Rep. 2012;39:3029–35.
Barr LF, Campbell SE, Bochner BS, Dang CV. Association of the decreased expression of alpha3beta1 integrin with the altered cell: environmental interactions and enhanced soft agar cloning ability of c-myc-overexpressing small cell lung cancer cells. Cancer Res. 1998;58:5537–45.
Zhang L, Qu J, Qi Y, Duan Y, Huang YW, Zhou Z, et al. EZH2 engages TGFβ signaling to promote breast cancer bone metastasis via integrin β1-FAK activation. Nat Commun. 2022;13:2543.
Davis-Lunn M, Goult BT, Andrews MR. Clutching at Guidance Cues: The Integrin-FAK Axis Steers Axon Outgrowth. Biology. 2023;12:954.
Brami-Cherrier K, Gervasi N, Arsenieva D, Walkiewicz K, Boutterin MC, Ortega A, et al. FAK dimerization controls its kinase-dependent functions at focal adhesions. Embo J. 2014;33:356–70.
Kleinschmidt EG, Schlaepfer DD. Focal adhesion kinase signaling in unexpected places. Curr Opin Cell Biol. 2017;45:24–30.
Patel MR, Infante JR, Moore KN, Keegan M, Poli A, Padval M, et al. Phase 1/1b study of the FAK inhibitor defactinib (VS-6063) in combination with weekly paclitaxel for advanced ovarian cancer. J Clin Oncol. 2014;32:5521.
Aung KL, McWhirter E, Welch S, Wang L, Lovell S, Stayner L-A, et al. A phase II trial of GSK2256098 and trametinib in patients with advanced pancreatic ductal adenocarcinoma (PDAC) (MOBILITY-002 Trial, NCT02428270). J Clin Oncol. 2018;36:409.
Mak G, Soria J-C, Blagden SP, Plummer R, Fleming RA, Nebot N, et al. A phase Ib dose-finding, pharmacokinetic study of the focal adhesion kinase inhibitor GSK2256098 and trametinib in patients with advanced solid tumours. Br J Cancer. 2019;120:975–81.
Lu Y, Sun H. Progress in the Development of Small Molecular Inhibitors of Focal Adhesion Kinase (FAK). J Med Chem. 2020;63:14382–403.
Chen B, Dragomir MP, Yang C, Li Q, Horst D, Calin GA. Targeting non-coding RNAs to overcome cancer therapy resistance. Signal Transduct Target Ther. 2022;7:121.
Guo X, Li M. LINC01089 is a tumor-suppressive lncRNA in gastric cancer and it regulates miR-27a-3p/TET1 axis. Cancer Cell Int. 2020;20:507.
Zhang H, Zhang H, Li X, Huang S, Guo Q, Geng D. LINC01089 functions as a ceRNA for miR-152-3p to inhibit non-small lung cancer progression through regulating PTEN. Cancer Cell Int. 2021;21:143.
Li M, Guo X. LINC01089 Blocks the Proliferation and Metastasis of Colorectal Cancer Cells via Regulating miR-27b-3p/HOXA10 Axis. Onco Targets Ther. 2020;13:8251–60.
Li S, Han Y, Liang X, Zhao M. LINC01089 inhibits the progression of cervical cancer via inhibiting miR-27a-3p and increasing BTG2. J Gene Med. 2021;23:e3280.
McGann JC, Oyer JA, Garg S, Yao H, Liu J, Feng X, et al. Polycomb- and REST-associated histone deacetylases are independent pathways toward a mature neuronal phenotype. Elife. 2014;3:e04235.
Wu Q, Guo J, Liu Y, Zheng Q, Li X, Wu C, et al. YAP drives fate conversion and chemoresistance of small cell lung cancer. Sci Adv. 2021;7:eabg1850.
Lim JS, Ibaseta A, Fischer MM, Cancilla B, O’Young G, Cristea S, et al. Intratumoural heterogeneity generated by Notch signalling promotes small-cell lung cancer. Nature. 2017;545:360–4.
Hong D, Knelson EH, Li Y, Durmaz YT, Gao W, Walton E, et al. Plasticity in the Absence of NOTCH Uncovers a RUNX2-Dependent Pathway in Small Cell Lung Cancer. Cancer Res. 2022;82:248–63.
Wang X, Hu X, Song W, Xu H, Xiao Z, Huang R, et al. Mutual dependency between lncRNA LETN and protein NPM1 in controlling the nucleolar structure and functions sustaining cell proliferation. Cell Res. 2021;31:664–83.
Xing YH, Yao RW, Zhang Y, Guo CJ, Jiang S, Xu G, et al. SLERT Regulates DDX21 Rings Associated with Pol I Transcription. Cell. 2017;169:664–78.e616.
Li X, Wang X, Song W, Xu H, Huang R, Wang Y, et al. Oncogenic Properties of NEAT1 in Prostate Cancer Cells Depend on the CDC5L-AGRN Transcriptional Regulation Circuit. Cancer Res. 2018;78:4138–49.
Acknowledgements
This work was supported by the National Key R&D Program of China (2019YFA0906000), the National Natural Science Foundation of China (82203459), China Postdoctoral Science Foundation (2023T160436), Guangdong Special Support Program (2021JC06Y578), the CSCO-Genecast Cancer Precision Therapy Research Fund (2019-056-ZZ), the Shenzhen Municipal Government of China (JCYJ20200109120016553, CJGJZD20200617102403009), the Sanming Project of Shenzhen Health and Family Planning Commission (SZSM202011017), Shenzhen High-level Hospital Construction Fund and the Shenzhen Institute of Synthetic Biology Scientific Research Program (ZTXM20214005), Shenzhen Portion of Shenzhen-Hong Kong Science and Technology Innovation Cooperation Zone (HTHZQSWS- KCCYB-2023060).
Author information
Authors and Affiliations
Contributions
XTW, XKL, LMN, and FL contributed equally to this work. XTW, XKL, FL, and LMN conducted experiments and wrote the manuscript. TG prepared and processed the clinical samples. BW, YSG, WRH, YLR, and XTW conceived the project. WRH, YLR, and BW supervised the study and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. All enrolled patients gave written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Li, X., Niu, L. et al. FAK-LINC01089 negative regulatory loop controls chemoresistance and progression of small cell lung cancer. Oncogene (2024). https://doi.org/10.1038/s41388-024-03027-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41388-024-03027-y