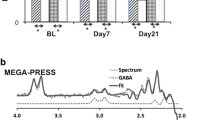
Abstract
Current treatments for adolescent alcohol use disorder (AUD) are mainly psychosocial and limited in their efficacy. As such, pharmacotherapies are being investigated as potential adjunctive treatments to bolster treatment outcomes. N-acetylcysteine is a promising candidate pharmacotherapy for adolescent AUD because of its tolerability and demonstrated ability to modulate glutamatergic, GABAergic, and glutathione systems. The primary objective of this double-blind, placebo-controlled, within-subjects crossover preliminary investigation was to measure potential changes within glutamate + glutamine (Glx), GABA, and glutathione levels in the dorsal anterior cingulate cortex (dACC) using proton magnetic resonance spectroscopy during 10-days of N-acetylcysteine (1200 mg twice daily) compared to 10-days of placebo in non-treatment seeking adolescents who use alcohol heavily (N = 31; 55% female). Medication adherence was confirmed via video. Effects on alcohol use were measured using Timeline Follow-Back as an exploratory aim. Linear mixed effects models controlling for baseline metabolite levels, brain tissue composition, alcohol use, cannabis use, and medication adherence found no significant differences in Glx, GABA, or glutathione levels in the dACC after N-acetylcysteine compared to placebo. There were also no measurable effects on alcohol use; however, this finding was underpowered. Findings were consistent in the subsample of participants who met criteria for AUD (n = 19). The preliminary null findings in brain metabolite levels may be due to the young age of participants, relatively low severity of alcohol use, and non-treatment seeking status of the population investigated. Future studies can use these findings to conduct larger, well-powered studies within adolescents with AUD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Lees B, Meredith LR, Kirkland AE, Bryant BE, Squeglia LM. Effect of alcohol use on the adolescent brain and behavior. Pharmacol Biochem Behav. 2020;192:172906.
World Health Organization. Global status report on alcohol and health 2018: World Health Organization; 2019.
Sun D, Adduru VR, Phillips RD, Bouchard HC, Sotiras A, Michael AM, et al. Adolescent alcohol use is linked to disruptions in age-appropriate cortical thinning: an unsupervised machine learning approach. Neuropsychopharmacology. 2022;11:1–10.
Lees B, Debenham J, Squeglia LM. Alcohol and cannabis use and the developing brain. Alcohol Res: Curr Rev. 2021;41:11.
Lees B, Mewton L, Stapinski LA, Squeglia LM, Rae CD, Teesson M. Neurobiological and cognitive profile of young binge drinkers: a systematic review and meta-analysis. Neuropsychol Rev. 2019;29:357–85.
Crews FT, Robinson DL, Chandler LJ, Ehlers CL, Mulholland PJ, Pandey SC, et al. Mechanisms of persistent neurobiological changes following adolescent alcohol exposure: NADIA consortium findings. Alcohol: Clin Exp Res. 2019;43:1806–22.
Gowin JL, Sloan ME, Stangl BL, Vatsalya V, Ramchandani VA. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry. 2017;174:1094–101.
Addolorato G, Vassallo GA, Antonelli G, Antonelli M, Tarli C, Mirijello A, et al. Binge drinking among adolescents is related to the development of alcohol use disorders: results from a cross-sectional study. Sci Rep. 2018;8:1–9.
Olsson CA, Romaniuk H, Salinger J, Staiger PK, Bonomo Y, Hulbert C, et al. Drinking patterns of adolescents who develop alcohol use disorders: results from the Victorian Adolescent Health Cohort Study. BMJ Open. 2016;6:e010455.
Ehlers CL, Stouffer GM, Gilder DA. Associations between a history of binge drinking during adolescence and self‐reported responses to alcohol in young adult Native and Mexican Americans. Alcohol: Clin Exp Res. 2014;38:2039–47.
Fadus MC, Squeglia LM, Valadez EA, Tomko RL, Bryant BE, Gray KM. Adolescent substance use disorder treatment: an update on evidence-based strategies. Curr Psychiatry Rep. 2019;21:1–10.
Jensen CD, Cushing CC, Aylward BS, Craig JT, Sorell DM, Steele RG. Effectiveness of motivational interviewing interventions for adolescent substance use behavior change: a meta-analytic review. J Consult Clin Psychol. 2011;79:433.
Tripodi SJ, Bender K, Litschge C, Vaughn MG. Interventions for reducing adolescent alcohol abuse: a meta-analytic review. Arch Pediatr Adolesc Med. 2010;164:85–91.
Tanner-Smith EE, Lipsey MW. Brief alcohol interventions for adolescents and young adults: a systematic review and meta-analysis. J Subst Abus Treat. 2015;51:1–18.
Chung T, Maisto SA. Relapse to alcohol and other drug use in treated adolescents: review and reconsideration of relapse as a change point in clinical course. Clin Psychol Rev. 2006;26:149–61.
Winters KC, Tanner-Smith EE, Bresani E, Meyers K. Current advances in the treatment of adolescent drug use. Adolesc Health, Med Ther. 2014;5:199.
Bridge JA, Iyengar S, Salary CB, Barbe RP, Birmaher B, Pincus HA, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297:1683–96.
Balog-Way DH, Evensen D, Löfstedt RE. Pharmaceutical benefit–risk perception and age differences in the USA and Germany. Drug Saf. 2020;43:1141–56.
O’Callaghan FV, Jordan N. Postmodern values, attitudes and the use of complementary medicine. Complement Ther Med. 2003;11:28–32.
Gray KM, Carpenter MJ, Baker NL, DeSantis SM, Kryway E, Hartwell KJ, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169:805–12.
Kalivas P, Volkow N. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16:974–86.
Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–13.
Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–72.
McClure EA, Gipson CD, Malcolm RJ, Kalivas PW, Gray KM. Potential role of N-acetylcysteine in the management of substance use disorders. CNS Drugs. 2014;28:95–106.
Slattery J, Kumar N, Delhey L, Berk M, Dean O, Spielholz C, et al. Clinical trials of N-acetylcysteine in psychiatry and neurology: a systematic review. Neurosci Biobehav Rev. 2015;55:294–321.
Lebourgeois S, González‐Marín MC, Jeanblanc J, Naassila M, Vilpoux C. Effect of N‐acetylcysteine on motivation, seeking and relapse to ethanol self‐administration. Addict Biol. 2018;23:643–52.
Quintanilla ME, Rivera‐Meza M, Berríos‐Cárcamo P, Salinas‐Luypaert C, Herrera‐Marschitz M, Israel Y. Beyond the “first hit”: marked inhibition by N‐acetyl cysteine of chronic ethanol intake but not of early ethanol intake. Parallel effects on ethanol‐induced saccharin motivation. Alcohol: Clin Exp Res. 2016;40:1044–51.
Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, et al. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci. 2007;27:13968–76.
Ramirez-Niño AM, D’Souza MS, Markou A. N-acetylcysteine decreased nicotine self-administration and cue-induced reinstatement of nicotine seeking in rats: comparison with the effects of N-acetylcysteine on food responding and food seeking. Psychopharmacology. 2013;225:473–82.
Olive MF, Cleva RM, Kalivas PW, Malcolm RJ. Glutamatergic medications for the treatment of drug and behavioral addictions. Pharmacol Biochem Behav. 2012;100:801–10.
Smaga I, Frankowska M, Filip M. N-acetylcysteine in substance use disorder: a lesson from preclinical and clinical research. Pharmacol Rep. 2021;73:1205–19.
Squeglia LM, Baker NL, McClure EA, Tomko RL, Adisetiyo V, Gray KM. Alcohol use during a trial of N-acetylcysteine for adolescent marijuana cessation. Addict Behav. 2016;63:172–7.
Squeglia LM, Tomko RL, Baker NL, McClure EA, Book GA, Gray KM. The effect of N-acetylcysteine on alcohol use during a cannabis cessation trial. Drug Alcohol Depend. 2018;185:17–22.
Ray LA, Bujarski S, Roche DJO, Magill M. Overcoming the “Valley of Death” in medications development for alcohol use disorder. Alcohol: Clin Exp Res. 2018;42:1612–22.
Seyhan AA. Lost in translation: the valley of death across preclinical and clinical divide–identification of problems and overcoming obstacles. Transl Med Commun. 2019;4:1–19.
Cohen-Gilbert JE, Jensen JE, Silveri MM. Contributions of magnetic resonance spectroscopy to understanding development: potential applications in the study of adolescent alcohol use and abuse. Dev Psychopathol. 2014;26:405–23.
Kohut SJ, Kaufman MJ. Magnetic resonance spectroscopy studies of substance use disorders: current landscape and potential future directions. Pharmacol Biochem Behav. 2021;200:173090.
Kirkland AE, Browning BD, Green R, Leggio L, Meyerhoff DJ, Squeglia LM. Brain metabolite alterations related to alcohol use: a meta-analysis of proton magnetic resonance spectroscopy studies. Mol Psychiatry. 2022;27:3223–36.
Bustillo JR. Use of proton magnetic resonance spectroscopy in the treatment of psychiatric disorders: a critical update. Dialogues Clin Neurosci. 2022;15:329–37.
Schmaal L, Veltman DJ, Nederveen A, Van Den Brink W, Goudriaan AE. N-acetylcysteine normalizes glutamate levels in cocaine-dependent patients: a randomized crossover magnetic resonance spectroscopy study. Neuropsychopharmacology/ 2012;37:2143–52.
Coles LD, Tuite PJ, Öz G, Mishra UR, Kartha RV, Sullivan KM, et al. Repeated‐dose oral N‐acetylcysteine in Parkinson’s disease: pharmacokinetics and effect on brain glutathione and oxidative stress. J Clin Pharmacol. 2018;58:158–67.
Tuura ROG, Warnock G, Ametamey S, Treyer V, Noeske R, Buck A, et al. Imaging glutamate redistribution after acute N-acetylcysteine administration: a simultaneous PET/MR study. NeuroImage. 2019;184:826–33.
Koob GF. A role for GABA mechanisms in the motivational effects of alcohol. Biochem Pharmacol. 2004;68:1515–25.
Koob GF. A role for GABA in alcohol dependence1. Adv Pharmacol. 2006;54:205–29.
Ozaras R, Tahan V, Aydin S, Uzun H, Kaya S, Senturk H. N-acetylcysteine attenuates alcohol-induced oxidative stress in the rat. World J Gastroenterol. 2003;9:125.
Schneider R, Bandiera S, Souza DG, Bellaver B, Caletti G, Quincozes-Santos A, et al. N-acetylcysteine prevents alcohol related neuroinflammation in rats. Neurochem Res. 2017;42:2135–41.
Liang J, Olsen RW. Alcohol use disorders and current pharmacological therapies: the role of GABA A receptors. Acta Pharmacol Sin. 2014;35:981–93.
Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27:113–24.
Uys JD, Mulholland PJ, Townsend DM. Glutathione and redox signaling in substance abuse. Biomed Pharmacother. 2014;68:799–807.
Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME. Monitoring the future national survey results on drug use, 1975-2020: overview, key findings on adolescent drug use. Institute for Social Research; 2021.
Johnston LD, O’Malley PM, Bachman JG. Monitoring the future: national results on adolescent drug use: overview of key findings. Focus. 2003;1:213–34.
Miech RA, Johnston LD, O’malley PM, Bachman JG, Schulenberg JE. Monitoring the future national survey results on drug use, 1975-2014. Volume 1, Secondary School Students. Institute for Social Research; 2015.
Squeglia LM, Sorg SF, Schweinsburg AD, Wetherill RR, Pulido C, Tapert SF. Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology. 2012;220:529–39.
Winters KC. Commentary on O’Brien: substance use disorders in DSM-V when applied to adolescents. Addiction. 2011;106:882.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33.
Sheehan DV, Sheehan KH, Shytle RD, Janavs J, Bannon Y, Rogers JE, et al. Reliability and validity of the mini international neuropsychiatric interview for children and adolescents (MINI-KID). J Clin Psychiatry 2010;71:17393.
Stoops WW, Strickland JC, Hays LR, Rayapati AO, Lile JA, Rush CR. Influence of n-acetylcysteine maintenance on the pharmacodynamic effects of oral ethanol. Pharmacol Biochem Behav. 2020;198:173037.
Lau-Barraco C, Braitman AL, Linden-Carmichael AN, Stamates AL. Differences in weekday versus weekend drinking among nonstudent emerging adults. Exp Clin Psychopharmacol. 2016;24:100.
Tomko RL, Gray KM, Oppenheimer SR, Wahlquist AE, McClure EA. Using REDCap for ambulatory assessment: Implementation in a clinical trial for smoking cessation to augment in-person data collection. Am J Drug Alcohol Abuse. 2019;45:26–41.
Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. Measuring alcohol consumption: Psychosocial and biochemical methods. 1992:41–72.
Mullins PG, Chen H, Xu J, Caprihan A, Gasparovic C. Comparative reliability of proton spectroscopy techniques designed to improve detection of J‐coupled metabolites. Magn Reson Med 2008;60:964–9.
Prisciandaro JJ, Mikkelsen M, Saleh MG, Edden RA. An evaluation of the reproducibility of 1H-MRS GABA and GSH levels acquired in healthy volunteers with J-difference editing sequences at varying echo times. Magn Reson Imaging. 2020;65:109–13.
Öngür D, Jensen JE, Prescot AP, Stork C, Lundy M, Cohen BM, et al. Abnormal glutamatergic neurotransmission and neuronal-glial interactions in acute mania. Biol Psychiatry. 2008;64:718–26.
Frye MA, Hinton DJ, Karpyak VM, Biernacka JM, Gunderson LJ, Feeder SE, et al. Anterior cingulate glutamate is reduced by acamprosate treatment in patients with alcohol dependence. J Clin Psychopharmacol. 2016;36:669.
Gruetter R, Tkáč I. Field mapping without reference scan using asymmetric echo‐planar techniques. Magn Reson Med. 2000;43:319–23.
Saleh MG, Rimbault D, Mikkelsen M, Oeltzschner G, Wang AM, Jiang D, et al. Multi-vendor standardized sequence for edited magnetic resonance spectroscopy. Neuroimage. 2019;189:425–31.
Oeltzschner G, Zöllner HJ, Hui SC, Mikkelsen M, Saleh MG, Tapper S, et al. Osprey: open-source processing, reconstruction & estimation of magnetic resonance spectroscopy data. J Neurosci Methods. 2020;343:108827.
Zöllner HJ, Tapper S, Hui SC, Barker PB, Edden RA, Oeltzschner G. Comparison of linear combination modeling strategies for edited magnetic resonance spectroscopy at 3 T. NMR Biomed. 2022;35:e4618.
Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1994;2:189–210.
Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, et al. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med. 2006;55:1219–26.
Smucny J, Carter CS, Maddock RJ. Medial prefrontal cortex glutamate is reduced in schizophrenia and moderated by measurement quality: a meta-analysis of 1H-MRS studies. Biol Psychiatry. 2021;90:643–51.
Erdfelder E, Faul F, Buchner A. GPOWER: a general power analysis program. Behav Res Methods Instrum Comput. 1996;28:1–11.
Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48.
Lüdecke D, Ben-Shachar MS, Patil I, Waggoner P, Makowski D. performance: an R package for assessment, comparison and testing of statistical models. J Open Source Softw. 2021;6:3139.
Van Buuren S, Groothuis-Oudshoorn K, Robitzsch A. Package ‘mice’: multivariate imputation by chained equations. CRAN Repos. 2019.
Ben-Shachar MS, Lüdecke D, Makowski D. effectsize: Estimation of effect size indices and standardized parameters. J Open Source Softw. 2020;5:2815.
Kunzetsova A, Brockhoff P, Christensen R. lmerTest package: tests in linear mixed effect models. J Stat Softw. 2017;82:1–26.
Schulte M, Goudriaan A, Kaag A, Kooi D, Van Den Brink W, Wiers R, et al. The effect of N-acetylcysteine on brain glutamate and gamma-aminobutyric acid concentrations and on smoking cessation: a randomized, double-blind, placebo-controlled trial. J Psychopharmacol. 2017;31:1377–9.
Holdiness MR. Clinical pharmacokinetics of N-acetylcysteine. Clin Pharmacokinet. 1991;20:123–34.
Marinkovic K, Myers ABA, Arienzo D, Sereno MI, Mason GF. Cortical GABA levels are reduced in young adult binge drinkers: association with recent alcohol consumption and sex. Neuroimage Clin. 2022;35:103091.
Haass‐Koffler CL, Piacentino D, Li X, Long VM, Lee MR, Swift RM, et al. Differences in sociodemographic and alcohol‐related clinical characteristics between treatment seekers and nontreatment seekers and their role in predicting outcomes in the COMBINE study for alcohol use disorder. Alcohol: Clin Exp Res. 2020;44:2097–108.
Ray LA, Bujarski S, Yardley MM, Roche DJ, Hartwell EE. Differences between treatment-seeking and non-treatment-seeking participants in medication studies for alcoholism: do they matter? Am J Drug Alcohol Abuse. 2017;43:703–10.
Meyerhoff D, Blumenfeld R, Truran D, Lindgren J, Flenniken D, Cardenas V, et al. Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcohol: Clin Exp Res. 2004;28:650–61.
Prisciandaro JJ, Schacht JP, Prescot AP, Brenner HM, Renshaw PF, Brown TR, et al. Intraindividual changes in brain GABA, glutamate, and glutamine during monitored abstinence from alcohol in treatment‐naive individuals with alcohol use disorder. Addict Biol. 2020;25:e12810.
Prisciandaro JJ, Schacht JP, Prescot AP, Renshaw PF, Brown TR, Anton RF. Brain glutamate, GABA, and glutamine levels and associations with recent drinking in treatment‐naive individuals with alcohol use disorder versus light drinkers. Alcohol: Clin Exp Res. 2019;43:221–6.
Prisciandaro JJ, Mellick W, Squeglia LM, Hix S, Arnold L, Tolliver BK. Results from a randomized, double‐blind, placebo‐controlled, crossover, multimodal‐MRI pilot study of gabapentin for co‐occurring bipolar and cannabis use disorders. Addict Biol. 2022;27:e13085.
Abdallah CG, Niciu MJ, Fenton LR, Fasula MK, Jiang L, Black A, et al. Decreased occipital cortical glutamate levels in response to successful cognitive-behavioral therapy and pharmacotherapy for major depressive disorder. Psychother Psychosom. 2014;83:298–307.
Machado-Vieira R, Zanetti MV, Otaduy MC, De Sousa RT, Soeiro-de-Souza MG, Costa AC, et al. Increased brain lactate during depressive episodes and reversal effects by lithium monotherapy in drug-naive bipolar disorder: a 3T 1H-MRS study. J Clin Psychopharmacol. 2017;37:40.
Bolton J, Moore GJ, MacMillan S, Stewart CM, Rosenberg DR. Case study: caudate glutamatergic changes with paroxetine persist after medication discontinuation in pediatric OCD. J Am Acad Child Adolesc Psychiatry. 2001;40:903–6.
Pollack MH, Jensen JE, Simon NM, Kaufman RE, Renshaw PF. High-field MRS study of GABA, glutamate and glutamine in social anxiety disorder: response to treatment with levetiracetam. Prog Neuro-Psychopharmacol Biol Psychiatry. 2008;32:739–43.
de la Fuente-Sandoval C, Reyes-Madrigal F, Mao X, Leon-Ortiz P, Rodriguez-Mayoral O, Jung-Cook H, et al. Prefrontal and striatal gamma-aminobutyric acid levels and the effect of antipsychotic treatment in first-episode psychosis patients. Biol Psychiatry. 2018;83:475–83.
Ueno F, Nakajima S, Iwata Y, Honda S, Torres‐Carmona E, Mar W, et al. GABA levels in the midcingulate cortex and clozapine response in patients with treatment‐resistant schizophrenia: a 1H‐MRS study. Psychiatry Clin Neurosci. 2022;76:587–94.
Back SE, McCauley JL, Korte KJ, Gros DF, Leavitt V, Gray KM, et al. A double-blind, randomized, controlled pilot trial of N-acetylcysteine in veterans with posttraumatic stress disorder and substance use disorders. J Clin Psychiatry. 2016;77:12406.
Choi IY, Andronesi OC, Barker P, Bogner W, Edden RA, Kaiser LG, et al. Spectral editing in 1H magnetic resonance spectroscopy: experts’ consensus recommendations. NMR Biomed. 2021;34:e4411.
Liu X-L, Li L, Li J-N, Rong J-H, Liu B, Hu Z-X. Reliability of glutamate quantification in human nucleus accumbens using proton magnetic resonance spectroscopy at a 70-cm wide-bore clinical 3T MRI system. Front Neurosci. 2017;11:686.
Gunn CM, Pankowska M, Harris M, Helsing E, Battaglia TA, Bagley SM. The representation of females in clinical trials for substance use disorder conducted in the United States (2010–19). Addiction. 2022;117:2583–90.
Funding
This work was supported by the National Institute of Alcohol and Alcoholism (NIAAA-K23-AA025399). AEK is supported by F32-AA029930-01A1, BDB is supported by T32DA007288, RG is supported by T32AA007474.
Author information
Authors and Affiliations
Contributions
All authors made significant contributions to this work. The project was conceived by LMS, KMG and RLT. LMS received funding for this project. DJM, JJP, RM, KTB and KMG were mentors for LMS K23. AMM, HL, BDB and AEK acquired data. AEK, PLF, and RG analyzed the data. The manuscript was written by AEK, BDB, RG, PLF, LMS and revised by all authors. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
KMG has provided consultation to Jazz Pharmaceuticals and has received research funding from Aelis Farma. RLT has received funding from the American Society of Addiction Medicine. The authors declare no other competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kirkland, A.E., Browning, B.D., Green, R. et al. N-acetylcysteine does not alter neurometabolite levels in non-treatment seeking adolescents who use alcohol heavily: A preliminary randomized clinical trial. Neuropsychopharmacol. 48, 1184–1193 (2023). https://doi.org/10.1038/s41386-023-01553-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01553-z