Abstract
Enhancing stress resilience in at-risk populations could significantly reduce the incidence of stress-related psychiatric disorders. We have previously reported that the administration of (R,S)-ketamine prevents stress-induced depressive-like behavior in male mice, perhaps by altering α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)-mediated transmission in hippocampal CA3. However, it is still unknown whether metabolites of (R,S)-ketamine can be prophylactic in both sexes. We administered (R,S)-ketamine or its metabolites (2R,6R)-hydroxynorketamine ((2R,6R)-HNK) and (2S,6S)-hydroxynorketamine ((2S,6S)-HNK) at various doses 1 week before one of a number of stressors in male and female 129S6/SvEv mice. Patch clamp electrophysiology was used to determine the effect of prophylactic drug administration on glutamatergic activity in CA3. To examine the interaction between ovarian hormones and stress resilience, female mice also underwent ovariectomy (OVX) surgery and a hormone replacement protocol prior to drug administration. (2S,6S)-HNK and (2R,6R)-HNK protected against distinct stress-induced behaviors in both sexes, with (2S,6S)-HNK attenuating learned fear in male mice, and (2R,6R)-HNK preventing stress-induced depressive-like behavior in both sexes. (R,S)-ketamine and (2R,6R)-HNK, but not (2S,6S)-HNK, attenuated large-amplitude AMPAR-mediated bursts in hippocampal CA3. All three compounds reduced N-methyl-D-aspartate receptor (NMDAR)-mediated currents 1 week after administration. Furthermore, ovarian-derived hormones were necessary for and sufficient to restore (R,S)-ketamine- and (2R,6R)-HNK-mediated prophylaxis in female mice. Our data provide further evidence that resilience-enhancing prophylactics may alter AMPAR-mediated glutamatergic transmission in CA3. Moreover, we show that prophylactics against stress-induced depressive-like behavior can be developed in a sex-specific manner and demonstrate that ovarian hormones are necessary for the prophylactic efficacy of (R,S)-ketamine and (2R,6R)-HNK in female mice.
Similar content being viewed by others
Introduction
MDD is the leading cause of disability worldwide, affecting over 300 million people, and often results from social, psychological, and biological factors [1]. In 80% of cases, a traumatic event triggers the first depressive episode, after which symptoms persist throughout an individual’s lifetime [2]. MDD is also highly comorbid with other psychiatric disorders, including post-traumatic stress disorder (PTSD), and approximately half of patients suffering from PTSD are concurrently diagnosed with depression [3,4,5]. Regardless of age or socioeconomic status, women are twice as likely as men to be diagnosed with depression and develop MDD earlier in life [1, 5]. fMRI data suggest that women experience fear more strongly than men and process trauma through distinct brain circuits [6]. Furthermore, certain antidepressants are less effective in women than in men, and changes in hormone levels can influence antidepressant efficacy in women [7, 8]. Given these sex-specific differences, it is necessary to develop more efficacious treatments for female patients.
Current treatments for MDD include lifestyle changes, cognitive behavioral therapy, and antidepressants [9]. However, these medications are slow to provide relief and fail to alleviate symptoms in up to 30% of patients [9]. These drawbacks have led to the use of (R,S)-ketamine, a commonly-used anesthetic, and (S)-ketamine (SpravatoTM) as rapid-acting antidepressants for treatment-resistant MDD (TRD) [10,11,12,13,14]. At sub-anesthetic doses, antidepressant (R,S)-ketamine has a rapid onset of 2 h in humans and 30 min in mice, can last up to 2 weeks in humans, and acts in a sex-specific manner in preclinical studies [14,15,16,17]. In mice, females are more sensitive than males to (R,S)-ketamine, requiring a lower dose to reverse depressive-like behaviors, and doses beneficial to males are depressogenic and anxiogenic in females [16]. These findings underscore the need for further sex-specific investigation into the use of (R,S)-ketamine in MDD treatment.
Because (R,S)-ketamine has a wide range of biological targets, isolating the specific mechanisms underlying its antidepressant actions has remained elusive [18]. Indeed, a wide variety of neurobiological mechanisms have been proposed to underlie (R,S)-ketamine’s efficacy as an antidepressant, many of which focus on the compound’s role as an N-methyl-D-aspartate receptor (NMDAR) inhibitor and include, but are not limited to (1) the disinhibition hypothesis, in which (R,S)-ketamine is proposed to preferentially inhibit NMDARs on inhibitory interneurons, leading to an overall increase in excitatory neurotransmission, (2) direct inhibition of the GluN2B subunit, (3) inhibition of spontaneous NMDAR activity, and (4) a reduction of burst firing in the lateral habenula (LHb) [19]. These proposed mechanisms are supported by evidence from a variety of studies, and, importantly, may work in concert to contribute to the antidepressant actions of (R,S)-ketamine [15, 19,20,21,22,23,24,25].
In addition to the proposed actions of the parent compound (R,S)-ketamine, recent studies have reported that stereospecific versions of the drug may play an important role in exerting antidepressant effects. (R,S)-ketamine is stereoselectively metabolized into various metabolites, including (R,S)-norketamine and (2R,6R;2S,6S)-hydroxynorketamine ((2R,6R;2S,6S)-HNK) [26]. (2R,6R;2S,6S)-HNK is a major metabolite found in the brain and plasma following (R,S)-ketamine infusion, comprising 15% of the original (R,S)-ketamine dose in humans [27]. The S(+)-enantiomers have a significantly higher affinity for the NMDAR than their R(−)-enantiomer counterparts, contributing to the development of (S)-ketamine (Spravato) as an FDA-approved treatment for TRD [12, 27]. However, recent data indicate that (R)-ketamine and (2R,6R)-HNK may exert more potent antidepressant efficacy with reduced psychotomimetic side effects, suggesting that NMDAR-independent mechanisms may play an important role in the antidepressant actions of (R,S)-ketamine [28,29,30]. Furthermore, while (2R,6R)-HNK is proposed to exert antidepressant effects independent of its parent compound, the data remain unclear [28, 31]. Thus, further investigation is needed to determine whether the (S)- or (R)-enantiomer contribute to the antidepressant actions of (R,S)-ketamine or (2R,6R;2S,6S)-HNK.
While many studies investigate (R,S)-ketamine’s actions in treating MDD, recent research indicate that (R,S)-ketamine could be used to prevent stress-induced depression before it develops. Our lab and others have shown that a single injection of (R,S)-ketamine before stress can protect against stress-induced depressive-like behavior and social avoidance as well as attenuate learned fear, suggesting the possibility of developing resilience-enhancing pharmacotherapy [32,33,34,35]. In addition, select studies in human subjects demonstrate that (R,S)-ketamine may prevent psychiatric disorders such as PTSD and post-partum depression (PPD), but the data in human populations remain unclear [36,37,38,39]. However, it is still unknown whether stereospecific (R,S)-ketamine metabolites, which lack the adverse side effects of their parent compound, can have the same prophylactic efficacy of their racemic precursor [28].
Here, we investigated whether stereospecific (R,S)-ketamine metabolites could have prophylactic efficacy in male and female mice. (R,S)-ketamine and (2S,6S)-HNK, but not (2R,6R)-HNK, attenuated learned fear in male mice. (R,S)-ketamine and (2R,6R)-HNK, but not (2S,6S)-HNK, reduced stress-induced depressive-like behavior in both sexes. Electrophysiological recordings revealed that the divergent behavioral actions of (2R,6R)-HNK and (2S,6S)-HNK corresponded with distinct effects on AMPAR- and NMDAR-mediated excitatory activity in hippocampal CA3. In female mice (R,S)-ketamine and (2R,6R)-HNK were prophylactically effective at a lower dose than in male mice. Moreover, we show that ovarian-derived hormones mediate the prophylactic actions of (R,S)-ketamine and (2R,6R)-HNK in female mice. These data emphasize the need for sex-specific approaches to the prevention and treatment of psychiatric disorders.
Materials and methods
Drugs
A single injection of saline (0.9% NaCl), (R,S)-ketamine (Fort Dodge Animal Health, Fort Dodge, IA), (2R,6R)-HNK (synthesized by the Organic Chemistry Collaborative Center (OCCC) at Columbia University), or (2S,6S)-HNK (synthesized by the OCCC) was administered ~8 weeks of age. (2S,6S)-HNK and (2R,6R)-HNK were synthesized from (S)-norketamine and (R)-norketamine, respectively, and structure was confirmed as previously described [28]. All drugs were prepared in physiological saline and administered intraperitoneally (i.p.) in volumes of 0.1 cc per 10 mg body weight.
All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the New York Psychiatric Institute (NYSPI) or by the European Directive, 2010/63/EU for the protection of laboratory animals, permissions # 92-256B, authorization ethical committee CEEA n°26 2012_098. For a full description of “Materials and methods”, please refer to the Supplementary Materials and Methods.
Results
(2R,6R)-HNK and (2S,6S)-HNK protect against distinct stress-induced behaviors in male 129S6/SvEv mice
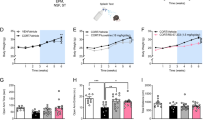
To determine whether (R,S)-ketamine metabolites are effective prophylactics, male 129S6/SvEv mice were administered saline, (R,S)-ketamine, (2R,6R)-HNK, or (2S,6S)-HNK at varying doses 1 week prior to 3-shock CFC and assessed in the forced swim test (FST) (Fig. 1a). (R,S)-ketamine dosing was chosen based on previous studies [32, 35]. During CFC training, neither (R,S)-ketamine nor (2R,6R)-HNK altered behavior, but multiple doses of (2S,6S)-HNK (0.025, 0.1, 0.3, 10, and 30 mg/kg) reduced freezing (Supplementary Fig. S1a-S1f). Upon re-exposure, (R,S)-ketamine (30 mg/kg) and (2S,6S)-HNK (0.025, 0.075, 0.1, 0.3, 10, and 30 mg/kg), but not (2R,6R)-HNK, reduced freezing (Fig. 1b–d).
a Experimental design. b–d (R,S)-ketamine (30 mg/kg) and (2S,6S)-HNK (0.025, 0.075, 0.1, 0.3, 10, and 30 mg/kg), but not (2R,6R)-HNK, attenuated learned fear in male mice. e–g (R,S)-ketamine (30 mg/kg) and (2R,6R)-HNK (0.075 mg/kg) decreased immobility time on day 2 of the FST. (2S,6S)-HNK did not alter immobility time on day 2 of the FST (n = 8–15 male mice per group). Error bars represent ± SEM. *p < 0.05. **p < 0.01. ***p < 0.0001. Sal, saline; K, (R,S)-ketamine; HNK, hydroxynorketamine; CFC, contextual fear conditioning; FST, forced swim test; min, minute; sec, second; mg, milligram; kg, kilogram.
The FST is widely used to quantify antidepressant efficacy in preclinical studies [40]. On day 1, overall immobility time was comparable between saline- and (R,S)-ketamine-administered mice but reduced in mice administered (2R,6R)-HNK (10 mg/kg) and (2S,6S)-HNK (0.075, 0.1, 0.3, 10, and 30 mg/kg) (Supplementary Fig. S1g-S1i). (2S,6S)-HNK at multiple doses reduced average immobility time (Supplementary Fig. S1j-S1l). On day 2, mice administered (R,S)-ketamine (30 mg/kg) and (2R,6R)-HNK (0.075 mg/kg), but not (2S,6S)-HNK, exhibited reduced immobility times compared with saline (Fig. 1e–g). Our results indicate, for the first time, that (2R,6R)-HNK and (2S,6S)-HNK prevent distinct stress-induced behaviors in male mice; specifically, (2S,6S)-HNK attenuates fear, while (2R,6R)-HNK decreases depressive-like behavior.
(2R,6R)-HNK and (2S,6S)-HNK differentially alter synaptic activity in CA3
To examine possible neurobiological effects contributing to the behavioral responses following drug administration, we performed whole-cell voltage clamp recordings of spontaneous excitatory postsynaptic currents (EPSCs) in pyramidal cells of hippocampal CA3 1 week after injection (Fig. 2a). We recently reported that prophylactic (R,S)-ketamine and prucalopride, a 5-HT4R agonist, attenuate bursts of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)-mediated synaptic currents despite targeting different receptors [41]. As previously demonstrated, saline-administered mice displayed these large AMPAR-mediated bursts and smaller-amplitude NMDAR-mediated EPSCs, which were revealed by bath application of 3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline (NBQX) (Fig. 2b, c). Both types of synaptic currents were attenuated in (R,S)-ketamine- and (2R,6R)-HNK-administered mice (Fig. 2d–g). (2S,6S)-HNK-administered mice also had decreased NMDAR-mediated EPSC amplitudes, but displayed bursts of AMPAR-mediated activity similar to controls (Fig. 2h, i). These changes occurred 1 week following administration when mice would typically be exposed to stress (Fig. 2j, k). Our data suggest that low-dose (2R,6R)-HNK and (2S,6S)-HNK differentially alter AMPAR-mediated transmission in hippocampal CA3, and that these actions are correlated with the drugs’ distinct behavioral effects.
a Experimental design. Male 129S6/SvEv mice were injected with saline, (R,S)-ketamine (30 mg/kg), (2R,6R)-HNK (0.075 mg/kg), or (2S,6S)-HNK (0.075 mg/kg). One week later, mice were sacrificed, and slice electrophysiology was performed in CA3 pyramidal cells. b Representative spontaneous synaptic currents in a saline-administered mouse from before (black) and after (red) bath application of the AMPAR blocker NBQX (20 μM), which revealed NMDAR-mediated currents (red) over a 20-s recording period (left). Insets (gray boxes) show an expanded view of a large-amplitude AMPAR-mediated burst and a small-amplitude NMDAR-mediated EPSC. c Scatter plot showing that a saline-administered mouse displayed large bursts of AMPAR-mediated EPSCs (498.9 ± 105.4 pA) and small-amplitude NMDAR-mediated currents (15.1 ± 2.1 pA). d Representative synaptic traces in a (R,S)-ketamine-administered mouse. Gray inset boxes show a single AMPAR-mediated EPSC, not a burst, and a smaller NMDA-mediated EPSC compared with saline-treated mouse. e In a mouse administered (R,S)-ketamine, large-amplitude AMPAR-mediated bursts were blocked and NMDAR-mediated EPSCs were attenuated. f Representative spontaneous currents in a (2R,6R)-HNK-administered mouse. Gray boxes indicate a single AMPAR-mediated current and an NMDAR-mediated EPSC smaller than the saline-treated mouse. g Similar to the (R,S)-ketamine-administered mouse, a mouse given (2R,6R)-HNK also had no large-amplitude AMPAR-mediated bursts and decreased NMDAR-mediated EPSC amplitudes. h Representative spontaneous currents in a (2S,6S)-HNK-administered mouse. Gray boxes indicate show an expanded view of an AMPA-mediated burst and a NMDA-mediated EPSC. i In mice administered (2S,6S)-HNK, NMDAR-mediated EPSCs, but not AMPAR-mediated bursts, were significantly reduced compared with saline-administered mice. j A Fisher’s exact test revealed a significant difference in AMPAR bursts between saline-administered and (R,S)-ketamine- or (2R,6R)-HNK-administered groups. Average amplitude of AMPAR bursts was comparable between mice administered saline and (2S,6S)-HNK. k Average amplitude of small NMDAR currents was significantly reduced in all experimental drug groups compared with saline-administered mice (n = 4–6 cells per group). Error bars represent ± SEM. Sal, saline; K, (R,S)-ketamine; HNK, hydroxynorketamine; CA3, Cornu Ammonis 3; pA, picoamps; NBQX, 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline; ms, millisecond; mg, milligram; kg, kilogram; EPSCs, excitatory postsynaptic currents; no., number.
Antidepressant efficacy of (R,S)-ketamine and (2R,6R)-HNK in male mice is strain-dependent
Previous results have demonstrated that (R,S)-ketamine and (2R,6R)-HNK are rapid-acting antidepressants in both sexes [16, 17, 28]. However, this effect is strain- and stress-specific [32, 42]. We, therefore, sought to replicate previous studies demonstrating antidepressant efficacy of (R,S)-ketamine and/or (2R,6R)-HNK in male 129S6/SvEv, C57BL/6J, or BALB/cJRJ mice [16, 43]. (R,S)-ketamine and (2R,6R)-HNK did not alter behavior in non-stressed male 129S6/SvEv mice, but reduced immobility time in non-stressed male C57BL/6J and BALB/cJRJ mice (Supplementary Fig. S2). These results support previous findings that (R,S)-ketamine and (2R,6R)-HNK exert strain-specific antidepressant effects [16, 43].
(R,S)-ketamine and (2R,6R)-HNK, but not (2S,6S)-HNK, are prophylactic against stress-induced depressive-like behavior in female 129S6/SvEv mice
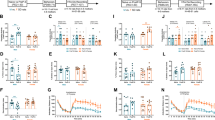
We next sought to determine whether (R,S)-ketamine, (2R,6R)-HNK, or (2S,6S)-HNK could be prophylactic in female mice. Female mice were administered saline, (R,S)-ketamine, (2R,6R)-HNK, or (2S,6S)-HNK at varying doses 1 week before CFC and administered the behavioral protocol outlined in Fig. 3a.
a Experimental design. b–d (R,S)-ketamine and (2R,6R)-HNK did not alter freezing during re-exposure to the CFC context. (2S,6S)-HNK (0.3 mg/kg) increased freezing during both re-exposure. e–g Administration of (R,S)-ketamine (10 mg/kg) and (2R,6R)-HNK (0.025 mg/kg) in female mice significantly reduced average immobility time on day 2 of the FST. (2S,6S)-HNK did not alter immobility time on day 2 of the FST. h–j (R,S)-ketamine (10 mg/kg) and (2R,6R)-HNK (0.025 mg/kg) did not alter distance traveled, distance traveled in the center, or time spent in the center of the OF compared with saline. k Similarly, nociception was not significantly affected by (R,S)-ketamine or (2R,6R)-HNK (n = 8–22 female mice per group). Error bars represent ± SEM. *p < 0.05. **p < 0.01. Sal, saline; K, (R,S)-ketamine; HNK, hydroxynorketamine; CFC, contextual fear conditioning; FST, forced swim test; OF, open field; TI, tail immersion test; min, minute; mg, milligram; kg, kilogram; sec, second; cm, centimeter.
(R,S)-ketamine and (2R,6R)-HNK did not alter fear behavior during CFC training or re-exposure (Supplementary Fig. S3a, b, d, e, Fig. 3b, c). (2S,6S)-HNK (0.3 mg/kg) increased freezing during both CFC training and re-exposure (Supplementary Fig. S3c, f, Fig. 3d). These data indicate that prophylactic (R,S)-ketamine and these metabolites do not attenuate learned fear in female mice. On FST day 1, all groups exhibited comparable immobility except mice administered (2S,6S)-HNK (0.3 mg/kg), which had higher overall immobility (Supplementary Fig. S3g–i). Average immobility time was comparable across all drug groups (Supplementary Fig. S3j–l). On FST day 2, (R,S)-ketamine (10 mg/kg) and (2R,6R)-HNK (0.025 mg/kg) reduced immobility time compared with saline (Fig. 3e–f). Immobility time in (2S,6S)-HNK-administered mice was not altered (Fig. 3g). Across groups, there was no significant change in behavior in the OF or TI tests, indicating that decreased immobility in the FST was not confounded by nonspecific effects on locomotion or nociception (Fig. 3h–k). These data show that female mice require ~1/3 of the dose needed by male mice to elicit the protective effects of (R,S)-ketamine and (2R,6R)-HNK.
Male and female rodents exhibit distinct pharmacokinetic profiles following (R,S)-ketamine administration [28]. To examine (R,S)-ketamine metabolism in female 129S6/SvEv mice, we used liquid-chromatography mass spectrometry (LC-MS) to quantify (R,S)-ketamine metabolite levels. Female mice were administered saline, (R,S)-ketamine (10 mg/kg), (2R,6R)-HNK (0.025 mg/kg), or (2S,6S)-HNK (0.025 mg/kg) and sacrificed 10 min later (Supplementary Fig. S4a). (R,S)-ketamine administration resulted in a comparable level of (2R,6R)-HNK and (2S,6S)-HNK in the brain and plasma (Supplementary Fig. S4b, c). In saline-, (2R,6R)-HNK-, or (2S,6S)-HNK-administered mice, the levels of these compounds were below quantification limits at the doses administered.
Next, we determined the effects of (R,S)-ketamine or (2R,6R)-HNK in non-stressed female mice. Mice were given saline, (R,S)-ketamine, or (2R,6R)-HNK 1 week before context exposure (Supplementary Fig. S5a). (R,S)-ketamine and (2R,6R)-HNK did not alter freezing (Supplementary Fig. S5b–d) or depressive-like behavior (Supplementary Fig. S5e–g), indicating that the behavioral effects of these compounds are specific to stress-induced behaviors.
(R,S)-ketamine and (2R,6R)-HNK prevent LH-induced depressive-like behavior in female 129S6/SvEv mice
We have previously shown that prophylactic (R,S)-ketamine attenuates helplessness in male mice [32]. To validate these findings in females, female mice were administered saline, (R,S)-ketamine, or (2R,6R)-HNK 1 week prior to LH training and subsequently tested in the FST and elevated plus maze (EPM) (Fig. 4a). During LH testing, there was no significant drug effect on session length or escape latency (Fig. 4b–d). However, (R,S)-ketamine and (2R,6R)-HNK significantly decreased immobility time in the FST compared with saline (Fig. 4e–g). In the EPM, mice in all groups traveled a comparable distance (Fig. 4h). (R,S)-ketamine, but not (2R,6R)-HNK, increased time in the open arms of the EPM compared with saline-administered mice (Fig. 4i–k, Supplementary Fig. S6a–c). These data indicate that, unlike in males, (R,S)-ketamine and (2R,6R)-HNK do not alter helpless behavior but decrease stress-induced depressive-like behavior, and that (R,S)-ketamine may be anxiolytic in females exposed to LH stress.
a Experimental design. b–d (R,S)-ketamine and (2R,6R)-HNK did not alter session length or escape latency compared with saline in the LH assay. e–g (R,S)-ketamine and (2R,6R)-HNK significantly reduced immobility on both days of the FST compared with saline. h Mice in all groups traveled a comparable distance in the EPM. i (R,S)-ketamine mice spent significantly less time in the closed arms of the EPM compared with saline mice. j Time spent in the open arms of the EPM was significantly increased in mice administered (R,S)-ketamine compared with saline-administered mice. k Entries into the open arms of the EPM was comparable in all groups. l Experimental design. m On day 1 of the FST, (2R,6R)-HNK-administered mice were significantly more immobile compared with saline mice. n, o On day 2 of the FST, (R,S)-ketamine and (2R,6R)-HNK reduced immobility compared with saline. p–r Freezing during CFC training and re-exposure was comparable across all drug groups (n = 5–10 mice per group). Error bars represent ± SEM. *p < 0.05. **p < 0.01. ***p < 0.0001. Sal, saline; K, (R,S)-ketamine; HNK, (2R,6R)-hydroxynorketamine; LH, learned helplessness; CIS, chronic immobilization stress; FST, forced swim test; CFC, contextual fear conditioning; EPM, elevated plus maze; min, minute; sec, second; mg, milligram; kg, kilogram; no., number; cm, centimeter.
(R,S)-ketamine and (2R,6R)-HNK are prophylactic against chronic stress in female 129S6/SvEv mice
As previously tested in male mice, we validated our findings in a model of chronic stress in female mice [32]. Mice were administered saline, (R,S)-ketamine, or (2R,6R)-HNK 1 week before a 10-day CIS protocol and tested in the FST and CFC (Fig. 4l). The order of behavioral tests was chosen to avoid confounding results in the FST with exposure to CFC stress. On FST day 1, there was no difference in immobility between saline- and (R,S)-ketamine-administered mice (Fig. 4m). (2R,6R)-HNK-administered mice exhibited increased immobility compared with saline-administered mice. However, on FST day 2, both drugs significantly lowered immobility compared with saline (Fig. 4n, o). During CFC training and re-exposure, freezing was comparable across all groups (Fig. 4p–r). These data indicate that prophylactic (R,S)-ketamine and (2R,6R)-HNK can protect against stress-induced depressive-like behavior following a chronic stressor.
(2R,6R)-HNK, but not (R,S)-ketamine, is prophylactic when administered 3 days before stress in female 129S6/SvEv mice
Previously, we determined that (R,S)-ketamine is prophylactic when administered 1 week, but not 1 day or 1 month, before stress in male mice [35]. To determine the optimal administration time in females, we tested if either compound could act prophylactically when given at a smaller time interval before stress (Supplementary Fig. S7a). Saline, (R,S)-ketamine (2.5, 10, or 30 mg/kg) or (2R,6R)-HNK (0.025 mg/kg) was administered 3 days before CFC in female mice. During CFC training, (R,S)-ketamine (10 mg/kg) increased freezing compared with saline (Supplementary Fig. S7b). During re-exposure, all groups froze at comparable levels (Supplementary Fig. S7c, d). In the FST, (2R,6R)-HNK reduced immobility compared with saline, indicating that (2R,6R)-HNK can be prophylactically effective 3 days before stress (Supplementary Fig. S7e–g).
We then determined whether (2R,6R)-HNK could be administered 24 h before stress (Supplementary Fig. S8). However, (2R,6R)-HNK did not attenuate learned fear or decrease depressive-like behavior, indicating that both (R,S)-ketamine and (2R,6R)-HNK are efficacious within a specific time window before stress.
Antidepressant efficacy of (R,S)-ketamine and (2R,6R)-HNK is sex- and stress-specific
Next, we tested whether (R,S)-ketamine and (2R,6R)-HNK could induce antidepressant-like behavioral effects in non-stressed female mice. Female 129S6/SvEv and C57BL/6NTac mice were administered saline, (R,S)-ketamine, or (2R,6R)-HNK 1 h prior to administration of the FST (Supplementary Fig. S9a, e). In both strains, immobility time was comparable across groups, indicating that (R,S)-ketamine (10 mg/kg) and (2R,6R)-HNK (10 mg/kg) do not exert antidepressant-like responses in stress-naive female mice (Supplementary Fig. S9b–d, f–h).
We then tested whether (R,S)-ketamine or (2R,6R)-HNK could be effective when administered after a stressor, as pre-exposure to stress can alter the antidepressant efficacy of (R,S)-ketamine (Supplementary Fig. S10a) [42]. Here, (R,S)-ketamine and (2R,6R)-HNK did not reduce immobility levels or alter fear behavior compared with saline (Supplementary Fig. S10b–f). These results suggest that (R,S)-ketamine and (2R,6R)-HNK do not induce antidepressant-like effects in 129S6/SvEv mice and may be dependent on the type of stress exposure in C57BL/6J mice.
Ovarian hormones mediate the prophylactic efficacy of (R,S)-ketamine and (2R,6R)-HNK in female 129S6/SvEv mice
We then hypothesized that increased drug sensitivity in female mice was dependent on ovarian-derived hormones. Female mice were ovariectomized (OVX) prior to injection of saline, (R,S)-ketamine, or (2R,6R)-HNK, and administered the behavioral protocol outlined in Fig. 5a. In sham and OVX groups, saline, (R,S)-ketamine, or (2R,6R-HNK) administration did not alter fear behavior (Fig. 5b–d). On FST day 1, in sham mice, (R,S)-ketamine reduced immobility time compared with saline (Fig. 5e). On FST day 2, in sham mice, both (R,S)-ketamine and (2R,6R)-HNK decreased immobility compared with saline controls. However, (R,S)-ketamine and (2R,6R)-HNK did not alter immobility in OVX mice (Fig. 5f, g), suggesting that ovarian-derived hormones are necessary for (R,S)-ketamine- and (2R,6R)-HNK-mediated prophylaxis in female mice.
a Experimental design. b–d Neither surgery nor drug administration significantly altered freezing behavior during CFC training or re-exposure. e On day 1 of the FST, sham + (R,S)-ketamine-administered mice had reduced immobility compared with sham + saline-administered mice. f, g On day 2 of the FST, within the sham group, (R,S)-ketamine and (2R,6R)-HNK reduced immobility compared with saline. However, within the OVX group, there was no effect of drug on depressive-like behavior in the FST. h Experimental design and figure legend. i–k Neither estrogen replacement, estrogen and cyclic progesterone replacement, nor prophylactic drug administration altered freezing during CFC training and re-exposure in OVX female mice. l On day 1 of the FST, E2 + (2R,6R)-HNK-administered mice exhibited significantly reduced immobility compared with Veh + (2R,6R)-HNK-administered mice. m, n On day 2 of the FST, immobility was comparable between all drug groups in the OVX + Veh group. In the OVX + E2 group, (2R,6R)-HNK-administered mice were significantly less immobile compared with saline-administered mice. In the OVX + E2/P4 group, both (R,S)-ketamine and (2R,6R)-HNK reduced immobility in the FST compared with saline-administered mice (n = 5–18 mice per group). Error bars represent ± SEM. *p < 0.05. **p < 0.01. OVX, ovariectomy; Sal, saline; K, (R,S)-ketamine; HNK, (2R,6R)-hydroxynorketamine; FST, forced swim test; min, minute; mg, milligram; kg, kilogram; sec, second; Veh, vehicle; E2, estrogen; P4, progesterone.
Finally, we investigated whether hormone replacement after OVX could restore the prophylactic effects of both drugs [44]. Female mice were ovariectomized and given 1 of 3 hormone replacement protocols prior to drug injection: (1) a subcutaneous vehicle (Veh) implant and Veh injections every fourth day (OVX + Veh), (2) a subcutaneous E2 implant and no injections (OVX + E2), or (3) a subcutaneous E2 implant and P4 injections every fourth day (OVX + E2/P4) (Fig. 5h). During CFC training and re-exposure, all groups exhibited comparable freezing (Fig. 5i–k). On FST day 1, OVX + E2 + (2R,6R)-HNK mice exhibited reduced immobility compared with the OVX + Veh + (2R,6R)-HNK group (Fig. 5l). On FST day 2, OVX + Veh control mice exhibited comparable immobility across all drug groups (Fig. 5m, n). In OVX + E2 mice, (2R,6R)-HNK reduced immobility compared with Sal. In OVX + E2/P4 mice, (R,S)-ketamine and (2R,6R)-HNK reduced immobility compared with saline. E2 or E2/P4 replacement alone did not alter depressive-like behavior in the FST. These data show that E2 alone can restore the prophylactic effects of (2R,6R)-HNK in OVX female mice, but not (R,S)-ketamine, while E2/P4 replacement restores the protective effects of both drugs.
Discussion
This series of experiments yielded 4 main findings: (1) (2S,6S)-HNK and (2R,6R)-HNK affect distinct stress-induced phenotypes in male mice; (2) (2S,6S)-HNK and (2R,6R)-HNK differentially alter glutamatergic activity in CA3; (3) (R,S)-ketamine and (2R,6R)-HNK are prophylactic against stress-induced depressive-like behavior, but do not alter learned fear, at smaller doses in female than in male mice; and (4) prophylactic efficacy of (R,S)-ketamine and (2R,6R)-HNK in female mice is modulated by ovarian hormones. (R,S)-ketamine and (2S,6S)-HNK attenuated learned fear in male, but not female, mice, while (R,S)-ketamine and (2R,6R)-HNK prevented stress-induced depressive-like behavior in both sexes. Effective doses of (R,S)-ketamine and (2R,6R)-HNK in female mice were 1/3 of the effective doses in male mice. Both (R,S)-ketamine and (2R,6R)-HNK protected against a variety of stressors, including CFC, LH, and CIS stress, in females. In male mice, (R,S)-ketamine and (2R,6R)-HNK, but not (2S,6S)-HNK robustly attenuated AMPAR-mediated bursts in hippocampal CA3. In female mice, ablation of ovarian hormones attenuated the prophylactic effects of both (R,S)-ketamine and (2R,6R)-HNK. However, replacement of estrogen alone restored the prophylactic properties of (2R,6R)-HNK, while replacement of both estrogen and progesterone restored the prophylactic actions of both (R,S)-ketamine and (2R,6R)-HNK. A graphical summary of behavioral results is included in Supplementary Table S1. Statistical analyses are included in Supplementary Table S2.
Our study is the first to demonstrate that stereospecific (R,S)-ketamine metabolites protect against distinct stress-induced behaviors. (2S,6S)-HNK attenuated learned fear only in male mice. (2R,6R)-HNK, however, prevented depressive-like behavior in both sexes. These behavioral actions were mirrored by the metabolites’ divergent effects on glutamatergic activity in hippocampal CA3, with (2S,6S)-HNK reducing NMDAR-mediated EPSCs, and (2R,6R)-HNK attenuating these synaptic currents as well as large AMPAR-mediated bursts 1 week after injection. Previous studies have also shown differing effects of stereospecific (R,S)-ketamine and (R,S)-ketamine metabolites on depressive-like behavior [12, 28,29,30, 45,46,47]. (S)-ketamine and its metabolites possess a greater affinity for the NMDAR than their (R)-enantiomers [27]. On the other hand, (2R,6R)-HNK may act preferentially on AMPARs, although previous evidence suggests that this action is dose-specific and may not directly alter depressive-like behavior [28, 48,49,50]. Our results suggest that manipulations targeting NMDAR-mediated neural circuits may be more efficacious in reducing fear behavior in males, while targeting AMPAR-mediated signaling may reduce depressive-like behavior in both sexes.
For translation into human populations, the mechanisms of resilience-enhancing prophylactics must be further elucidated. In male mice, we have shown that prophylactic (R,S)-ketamine acts on the transcription factor ΔFosB to alter neural ensembles in ventral CA3 and changes the excitatory/inhibitory balance of neurotransmitters in the brain after stress [51, 52]. Other studies indicate that (R,S)-ketamine may sensitize inhibitory dorsal raphe nucleus (DRN)-projecting neurons in prelimbic cortext to exert its protective effects [33, 34]. Our laboratory recently demonstrated that prophylactic (R,S)-ketamine and prucalopride, a 5-HT4R agonist, attenuate AMPAR-mediated synaptic bursts in CA3, despite targeting different receptors [41]. Here, we show that, similarly to (R,S)-ketamine, (2R,6R)-HNK, but not (2S,6S)-HNK, decreases large-amplitude AMPAR-mediated bursts in CA3. Moreover, a recent study showed that (2R,6R)-HNK enhances pre-synaptic NMDAR-independent glutamatergic transmission at Schaffer collateral terminals in CA1 [53]. Combined, these results suggest that altering AMPAR-mediated glutamatergic transmission along the Schaffer collateral pathway at CA3-CA1 synapses may contribute to the modulation of resilience against stress-induced depressive-like behavior. Further studies examining these and other resilience-enhancing mechanisms will be crucial for the development of prophylactic agents.
Our data indicate that extremely small doses of (2R,6R)-HNK can alter neural activity and behavior despite resulting in below-quantification levels following administration. Due to technical limitations, LC-MS may not be sensitive enough to detect concentrations of (2R,6R)-HNK within the picogram range, and future studies may have more success using accelerated mass spectrometry [54, 55]. To our knowledge, microdoses of (R,S)-ketamine and its metabolites have not previously been studied. However, these doses are more comparable to drug levels observed in humans following administration of antidepressant (R,S)-ketamine [50]. Evidence suggests that studying microdosing in preclinical models may lead to more reliable predictions of human pharmacokinetics [56,57,58,59]. In addition, a separate type of microdosing, in which individuals repeatedly ingest small quantities of psychedelic substances, is reported to exert cognitive benefits without inducing psychotropic or addictive side effects [54,55,56,57,58,59,60,61,62]. However, further study is necessary to determine how microdoses of (2R,6R)-HNK can effectively enhance stress resilience.
Historically, females have not been used to study pharmacological therapies for psychiatric disorders due to concerns over behavioral variability. However, male and female subjects respond differently to (R,S)-ketamine, perhaps due to differences in pharmacokinetics or pharmacodynamics [16, 17, 63,64,65]. Another potential factor is the estrous cycle. Because of our lengthy experimental manipulations, we did not track the estrous cycle in our study. Nevertheless, we found that replacing E2/P4 or E2 alone in OVX mice differentially restored the prophylactic efficacy of (R,S)-ketamine and (2R,6R)-HNK. These results support previous work demonstrating that cyclic E2/P4 treatment restores hedonic response to (R,S)-ketamine in OVX female rats [66]. This study also showed that (R,S)-ketamine administration in combination with E2/P4 treatment, but not E2 or P4 alone, upregulates brain-derived neurotrophic factor (BDNF) expression in the hippocampus (HPC) [66]. Combined with our findings, these results suggest that prophylactic (R,S)-ketamine and (2R,6R)-HNK may differentially modulate BDNF levels to enhance resilience against stress-induced depressive-like behavior. However, further study is necessary to determine how interactions between ovarian hormones and (R,S)-ketamine and/or its metabolites may protect against stress-induced behaviors.
In addition, we observed dissimilarities in prophylactic (R,S)-ketamine’s effect on fear behavior between the sexes. A recent study reported that prophylactic (R,S)-ketamine administered to female rats before LH prevented reductions in social exploration but did not report changes in escape behavior [32, 34]. Indeed, across species, many studies have demonstrated sex-specific divergent symptoms of mood disorders. While women diagnosed with MDD experience higher rates of comorbid anxiety disorders and greater suicidal ideation, men are at greater risk of comorbid substance abuse [67]. In rodents, females do not acquire a LH phenotype, do not express anhedonia as robustly as males, and are more susceptible to behavioral changes during swimming stress [68, 69]. Thus, males and females likely process stress using separate neural strategies that result in distinct behavioral responses. Consequently, many paradigms developed to model pathological behavior in male animals may be inappropriate for use in females.
Previous studies have shown that the antidepressant effects of (R,S)-ketamine and (2R,6R)-HNK in both sexes are strain- and stress-specific [15, 28, 29, 42]. Previous reports show that testing mice of different genetic backgrounds in the same behavioral assays can lead to opposing conclusions, suggesting that future experiments should include a variety of mouse strains to determine antidepressant efficacy [70]. Importantly, we replicated two different studies in male C57BL/6J and BALB/cJ mice and found that previously-tested doses of (R,S)-ketamine and (2R,6R)-HNK exerted antidepressant effects [16, 43]. Although our data did not indicate antidepressant efficacy in female mice, our results may be specific to the drug doses, mouse strains, or behavioral paradigms we utilized. Moreover, we assessed depressive-like behaviors using the FST; however, future studies should include additional assays to assess anhenodia. These data highlight the importance of utilizing numerous mouse strains and behavioral tests to infer drug-behavioral relationships.
In summary, this study demonstrates that stereospecific metabolites of (R,S)-ketamine induce distinct behavioral phenotypes in a sex-specific manner perhaps by differentially altering glutamatergic activity or acting on sex-specific hormones. Our experiments offer insight into sex-specific mechanisms underlying resilience to stress against depressive-like behavior. Ultimately, these studies will elucidate the underlying sex-specific neuropathology of MDD and contribute to advancements in targeted therapies for stress-related disorders.
Funding and disclosure
BKC was supported by Neurobiology & Behavior Research Training Grant T32 HD007430-19 and the Coulter Biomedical Accelerator (BioMedX) program. VML was supported by an NIA K01 AG054765. CTL was supported by an NIH DP5 OD017908. AS was supported by the I.I. Rabi Scholars Program at Columbia University. CAD was supported by an NIH DP5 OD017908, a New York Stem Cell Science NYSTEM C-021957, and a gift from For the Love of Travis, Inc. CTL, XX, SD, RFS, TBC, and DWL reported no potential conflicts of interests. BKC, RAB, IM-D, DJD, AMG, and CAD are named on provisional and non-provisional patent applications for the prophylactic use of (R,S)-ketamine and related compounds against stress-related psychiatric disorders.
References
WHO. Depression fact sheet. 2018.
(US) NRC, Institute of Medicine Committee on Depression PP, and the Healthy Development of Children. Depression in parents, parenting, and children: opportunities to improve identification, treatment, and prevention. Washington, D.C.: National Academies Press (US); 2009.
Flory JD, Yehuda R. Comorbidity between post-traumatic stress disorder and major depressive disorder: alternative explanations and treatment considerations. Dialogues Clin Neurosci. 2015;17:141–50.
Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602.
Piccinelli M, Wilkinson G. Gender differences in depression. Critical review. Br J Psychiatry. 2000;177:486–92.
Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–74.
Kornstein SG. Gender differences in depression: implications for treatment. J Clin Psychiatry. 1997;58:12–8.
Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, et al. Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry. 2000;157:1445–52.
Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 2012;6:369–88.
Serafini G, Howland RH, Rovedi F, Girardi P, Amore M. The role of ketamine in treatment-resistant depression: a systematic review. Curr Neuropharmacol. 2014;12:444–61.
Al Shirawi MI, Kennedy SH, Ho KT, Byrne R, Downar J. Oral ketamine in treatment-resistant depression: a clinical effectiveness case series. J Clin Psychopharmacol. 2017;37:464–67.
Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75:139–48.
Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–42.
Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64.
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–5.
Franceschelli A, Sens J, Herchick S, Thelen C, Pitychoutis PM. Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naive and “depressed” mice exposed to chronic mild stress. Neuroscience. 2015;290:49–60.
Carrier N, Kabbaj M. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology. 2013;70:27–34.
Browne CA, Lucki I. Antidepressant effects of ketamine: mechanisms underlying fast-acting novel antidepressants. Front Pharmacol. 2013;4:161.
Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23:801–11.
Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickar D. Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Psychiatry. 1997;154:805–11.
Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–27.
Nosyreva E, Szabla K, Autry AE, Ryazanov AG, Monteggia LM, Kavalali ET. Acute suppression of spontaneous neurotransmission drives synaptic potentiation. J Neurosci. 2013;33:6990–7002.
Miller OH, Yang L, Wang C-C, Hargroder EA, Zhang Y, Delpire E, et al. GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. eLife. 2014;3:e03581–e81.
Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, et al. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature. 2018;554:317–22.
Duman RS, Li N, Liu R-J, Duric V, Aghajanian G. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62:35–41.
Zarate CA Jr., Brutsche N, Laje G, Luckenbaugh DA, Venkata SL, Ramamoorthy A, et al. Relationship of ketamine’s plasma metabolites with response, diagnosis, and side effects in major depression. Biol Psychiatry. 2012;72:331–8.
Mion G, Villevieille T. Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings). CNS Neurosci Ther. 2013;19:370–80.
Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–6.
Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, et al. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5:e632.
Zhang JC, Li SX, Hashimoto KR. (−)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharm Biochem Behav. 2014;116:137–41.
Yamaguchi JI, Toki H, Qu Y, Yang C, Koike H, Hashimoto K, et al. (2R,6R)-Hydroxynorketamine is not essential for the antidepressant actions of (R)-ketamine in mice. Neuropsychopharmacology. 2018;43:1900–07.
Brachman RA, McGowan JC, Perusini JN, Lim SC, Pham TH, Faye C, et al. Ketamine as a prophylactic against stress-induced depressive-like behavior. Biol Psychiatry. 2016;79:776–86.
Amat J, Dolzani SD, Tilden S, Christianson JP, Kubala KH, Bartholomay K, et al. Previous ketamine produces an enduring blockade of neurochemical and behavioral effects of uncontrollable stress. J Neurosci. 2016;36:153–61.
Dolzani SD, Baratta MV, Moss JM, Leslie NL, Tilden SG, Sorensen AT, et al. Inhibition of a descending prefrontal circuit prevents ketamine-induced stress resilience in females. eNeuro. 2018;5:ENEURO.0025–18.2018.
McGowan JC, LaGamma CT, Lim SC, Tsitsiklis M, Neria Y, Brachman RA, et al. Prophylactic ketamine attenuates learned fear. Neuropsychopharmacology. 2017;42:1577–89.
McGhee LL, Maani CV, Garza TH, Slater TM, Petz LN, Fowler M. The intraoperative administration of ketamine to burned U.S. service members does not increase the incidence of post-traumatic stress disorder. Mil Med. 2014;179:41–6.
Ma J-H, Wang S-Y, Yu H-Y, Li D-Y, Luo S-C, Zheng S-S, et al. Prophylactic use of ketamine reduces postpartum depression in Chinese women undergoing cesarean section. Psychiatry Res. 2019;279:252–58.
Xu Y, Li Y, Huang X, Chen D, She B, Ma D. Single bolus low-dose of ketamine does not prevent postpartum depression: a randomized, double-blind, placebo-controlled, prospective clinical trial. Arch Gynecol Obstet. 2017;295:1167–74.
McGhee LL, Maani CV, Garza TH, Gaylord KM, Black IH. The correlation between ketamine and posttraumatic stress disorder in burned service members. J Trauma. 2008;64:S195–8.
Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–2.
Chen BK, Mendez-David I, Luna VM, Faye C, Gardier AM, David DJ, et al. Prophylactic efficacy of 5-HT(4)R agonists against stress. Neuropsychopharmacology. 2019. https://doi.org/10.1038/s41386-019-0540-3.
Fitzgerald PJ, Yen JY, Watson BO. Stress-sensitive antidepressant-like effects of ketamine in the mouse forced swim test. PLoS ONE. 2019;14:e0215554.
Pham TH, Defaix C, Xu X, Deng S-X, Fabresse N, Alvarez J-C, et al. Common neurotransmission recruited in (R,S)-ketamine and (2R,6R)-hydroxynorketamine–induced sustained antidepressant-like effects. Biol Psychiatry. 2018;84:e3–e6.
Rissman EF, Early AH, Taylor JA, Korach KS, Lubahn DB. Estrogen receptors are essential for female sexual receptivity. Endocrinology. 1997;138:507–10.
Pham TH, Defaix C, Xu X, Deng SX, Fabresse N, Alvarez JC, et al. Common neurotransmission recruited in (R,S)-ketamine and (2R,6R)-hydroxynorketamine-induced sustained antidepressant-like effects. Biol Psychiatry. 2018;84:e3–e6.
Singh JB, Fedgchin M, Daly E, Xi L, Melman C, De Bruecker G, et al. Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry. 2016;80:424–31.
Fukumoto K, Toki H, Iijima M, Hashihayata T, Yamaguchi JI, Hashimoto K, et al. Antidepressant potential of (R)-ketamine in rodent models: comparison with (S)-ketamine. J Pharm Exp Ther. 2017;361:9–16.
Aleksandrova LR, Phillips AG, Wang YT. Antidepressant effects of ketamine and the roles of AMPA glutamate receptors and other mechanisms beyond NMDA receptor antagonism. J Psychiatry Neurosci. 2017;42:222–9.
Suzuki K, Nosyreva E, Hunt KW, Kavalali ET, Monteggia LM. Effects of a ketamine metabolite on synaptic NMDAR function. Nature. 2017;546:E1–e3.
Shaffer CL, Dutra JK, Tseng WC, Weber ML, Bogart LJ, Hales K, et al. Pharmacological evaluation of clinically relevant concentrations of (2R,6R)-hydroxynorketamine. Neuropharmacology. 2019;153:73–81.
McGowan JC, Hill C, Mastrodonato A, LaGamma CT, Kitayev A, Brachman RA, et al. Prophylactic ketamine alters nucleotide and neurotransmitter metabolism in brain and plasma following stress. Neuropsychopharmacology. 2018;43:1813–21.
Mastrodonato A, Martinez R, Pavlova IP, LaGamma CT, Brachman RA, Robison AJ, et al. Ventral CA3 activation mediates prophylactic ketamine efficacy against stress-induced depressive-like behavior. Biol Psychiatry. 2018;84:846–56.
Riggs LM, Aracava Y, Zanos P, Fischell J, Albuquerque EX, Pereira EFR. et al. 2R,6R)-hydroxynorketamine rapidly potentiates hippocampal glutamatergic transmission through a synapse-specific presynaptic mechanism. Neuropsychopharmacology. 2020;45:426–36.
Burt T, John CS, Ruckle JL, Vuong LT. Phase-0/microdosing studies using PET, AMS, and LC-MS/MS: a range of study methodologies and conduct considerations. Accelerating development of novel pharmaceuticals through safe testing in humans—a practical guide. Expert Opin Drug Deliv. 2017;14:657–72.
Wotherspoon AT, Safavi-Naeini M, Banati RB. Microdosing, isotopic labeling, radiotracers and metabolomics: relevance in drug discovery, development and safety. Bioanalysis. 2017;9:1913–33.
Lappin G. The expanding utility of microdosing. Clin Pharm Drug Dev. 2015;4:401–6.
Burt T, Yoshida K, Lappin G, Vuong L, John C, de Wildt SN, et al. Microdosing and other phase 0 clinical trials: facilitating translation in drug development. Clin Transl Sci. 2016;9:74–88.
Bosgra S, Vlaming ML, Vaes WH. To apply microdosing or not? Recommendations to single out compounds with non-linear pharmacokinetics. Clin Pharmacokinet. 2016;55:1–15.
Lappin G, Noveck R, Burt T. Microdosing and drug development: past, present and future. Expert Opin Drug Metab Toxicol. 2013;9:817–34.
Polito V, Stevenson RJ. A systematic study of microdosing psychedelics. PLoS ONE 2019;14:e0211023.
Fadiman J, Korb S. Might microdosing psychedelics be safe and beneficial? An initial exploration. J Psychoact Drugs. 2019;51:118–22.
Prochazkova L, Lippelt DP, Colzato LS, Kuchar M, Sjoerds Z, Hommel B. Exploring the effect of microdosing psychedelics on creativity in an open-label natural setting. Psychopharmacol (Berl). 2018;235:3401–13.
Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009;48:143–57.
Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol. 2009;76:215–28.
Schwartz JB. The influence of sex on pharmacokinetics. Clin Pharmacokinet. 2003;42:107–21.
Saland SK, Schoepfer KJ, Kabbaj M. Hedonic sensitivity to low-dose ketamine is modulated by gonadal hormones in a sex-dependent manner. Sci Rep. 2016;6:21322.
Altemus M, Sarvaiya N, Neill Epperson C. Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol. 2014;35:320–30.
Dalla C, Edgecomb C, Whetstone AS, Shors TJ. Females do not express learned helplessness like males do. Neuropsychopharmacology. 2008;33:1559–69.
Kokras N, Dalla C. Sex differences in animal models of psychiatric disorders. Br J Pharm. 2014;171:4595–619.
Sittig LJ, Carbonetto P, Engel KA, Krauss KS, Barrios-Camacho CM, Palmer AA. Genetic background limits generalizability of genotype-phenotype relationships. Neuron. 2016;91:1253–59.
Acknowledgements
We thank M. Grunebaum, S. Ramirez, B. McEwen, and members of the laboratory for insightful comments on this project and manuscript. In addition, we thank Dr. Moshe Shalev, Dr. Girma Asfaw, and Lisa Moyano for assistance in performing ovariectomies.
Author information
Authors and Affiliations
Contributions
BKC and CAD conceived and designed the experiments. XX, SD, and DWL provided pharmacological agents. BKC, CTL, AS, RAB, IM-D, DJD, and AMG performed behavioral experiments and analysis. VML performed electrophysiological experiments. RFS and TBC performed mass spectrometry experiments. BKC and CAD wrote the paper in consultation with all other authors.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, B.K., Luna, V.M., LaGamma, C.T. et al. Sex-specific neurobiological actions of prophylactic (R,S)-ketamine, (2R,6R)-hydroxynorketamine, and (2S,6S)-hydroxynorketamine. Neuropsychopharmacol. 45, 1545–1556 (2020). https://doi.org/10.1038/s41386-020-0714-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-020-0714-z
This article is cited by
-
Hydroxynorketamine, but not ketamine, acts via α7 nicotinic acetylcholine receptor to control presynaptic function and gene expression
Translational Psychiatry (2024)
-
BDNF-TrkB signaling-mediated upregulation of Narp is involved in the antidepressant-like effects of (2R,6R)-hydroxynorketamine in a chronic restraint stress mouse model
BMC Psychiatry (2022)
-
Post-traumatic stress disorder: clinical and translational neuroscience from cells to circuits
Nature Reviews Neurology (2022)
-
The role of BDNF in mediating the prophylactic effects of (R,S)-ketamine on fear generalization and extinction
Translational Psychiatry (2022)
-
Target deconvolution studies of (2R,6R)-hydroxynorketamine: an elusive search
Molecular Psychiatry (2022)