Abstract
Patients with major depressive disorder (MDD) often have structural and functional deficits in the ventromedial prefrontal cortex (vmPFC), but the underlying molecular pathways are incompletely understood. The neuropeptide precursor VGF (non-acronymic) plays a critical role in depression and antidepressant efficacy in hippocampus and nucleus accumbens, however its function in vmPFC has not been investigated. Here, we show that VGF levels were reduced in Brodmann area 25 (a portion of human vmPFC) of MDD patients and in mouse vmPFC following chronic restraint stress (CRS), and were increased by ketamine in mouse vmPFC. VGF overexpression in vmPFC prevented behavioral deficits induced by CRS, and VGF knockdown in vmPFC increased susceptibility to subchronic variable stress (SCVS) and reduced ketamine’s antidepressant efficacy. Acute intra-vmPFC TLQP-62 infusion induced behavioral phenotypes that mimic those produced by antidepressant drug treatment. These antidepressant-like effects were sustained for 7 days and were abolished by local Bdnf gene ablation, or pretreatment with xestospongin C, an inhibitor of IP3-mediated Ca2+ release, or SKF96365, an inhibitor of store-operated and TRPC channel-mediated Ca2+ entry. In conclusion, VGF in the vmPFC regulates susceptibility to stress and the antidepressant response to ketamine. TLQP-62 infusion produces sustained antidepressant responses that require BDNF expression and calcium mobilization in vmPFC.
Similar content being viewed by others
Introduction
Major depressive disorder (MDD) is a highly prevalent mental illness [1] with limited effective treatment options available. The ventromedial prefrontal cortex (vmPFC) is an important component of brain circuits that regulate affective behaviors [2]. Reduced volume in subgenual anterior cingulate cortex (sgACC; a portion of human vmPFC), which includes Brodmann area (BA) 25 posteriorly and parts of BA24 and BA32 anteriorly [3] has been observed in depressed patients [4], and spine loss and decreased dendritic arborization in the vmPFC (including prelimbic and infralimbic cortexes) have been reported in rodents following chronic stress exposure [5]. Recent preclinical studies also revealed that vmPFC stimulation [6] and lesion [7] induce antidepressant and prodepressant effects in rodents, respectively.
VGF (non-acronymic) is a neuropeptide precursor that is robustly regulated by neurotrophic growth factors [8], including brain-derived neurotrophic factor (BDNF). Stress exposure in rodents and MDD in humans decrease and increase VGF expression in hippocampus and nucleus accumbens, respectively [9, 10]. VGF has antidepressant efficacy in hippocampus while it is pro-depressant in nucleus accumbens [10]. Exercise or treatment with antidepressants, including ketamine and imipramine, increases VGF expression in hippocampus [9,10,11]. VGF-derived carboxy-terminal peptides TLQP-62 and AQEE-30 (named by 4 N-terminal amino acids and length), administered via intra-hippocampal or intracerebroventricular infusion, produce antidepressant effects [9,10,11], while TLQP-62 additionally enhances memory formation [12, 13], potentially by influencing synaptic plasticity and neurogenesis [14, 15]. Previous studies implicate the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor subunit GluR1 in the pathophysiology of depression [16, 17]. Interestingly, our recent study found that intrahippocampal TLQP-62 infusion promotes GluR1 phosphorylation (Ser845) and increases synaptic GluR1 abundance in a rapamycin-sensitive manner, suggesting a potential molecular mechanism underlying the antidepressant actions of TLQP-62 [10].
Reduced VGF expression has been reported in sgACC of human subjects with MDD [18]. In the present study, we sought to determine whether VGF expression is regulated in a specific sub-region of human sgACC (BA25) and whether rodent vmPFC VGF/TLQP-62 regulates depression-related behaviors and response to ketamine treatment by influencing vmPFC GluR1 levels.
Materials and Methods
Animals
Generation of Vgfflpflox/flpflox (abbreviated here Vgfff/ff) mice and breeding scheme has been previously described [12]. C57BL/6 J, Vgfff/ff, BDNF floxed [19] (designated Bdnfflox/flox, BL6/sv129 background; generously provided by Dr. Eric Nestler) at 2–4 months of age were housed on a 12 h light-dark cycle with ad libitum access to food and water. All mice were single housed at least 3 days prior to the start of behavioral experiments. All mouse studies were conducted in accordance with the U.S. National Institutes of Health Guidelines for the Care and Use of Experimental Animals, using protocols approved by the Institutional Animal Care and Use Committee of the Icahn School of Medicine at Mount Sinai.
Stereotaxic viral and peptide infusion
Vgfff/ff, Bdnfflox/flox, or wild-type mice at 2–4 months of age were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg). Thirty-three gauge syringe needles (Hamilton, Reno, Nevada) were used to bilaterally infuse 0.5 μl of Adeno-Associated Virus (AAV) or peptide into mouse vmPFC (AP = + 1.85, ML = ±0.8, and DV = −2.3 from Bregma (mm) at 10°) at a rate of 0.1 μl per min. The needle remained in place for 5 min before removal to prevent backflow. AAV-CreGFP and AAV-GFP (AAV2 genotype, AAV2 serotype), and AAV5-GFP and AAV5-VGF (mouse VGF cDNA in pTR-UF12, AAV5 serotype), were prepared by the Vector Core at the University of North Carolina at Chapel Hill. Peptides TLQP-62 and its scrambled control SC-62 were synthesized by GenScript (Piscataway, New Jersey). Mice were allowed to recover for 21 days before behavioral testing.
Chronic restraint stress
Chronic restraint stress (CRS) was performed as previously described [20]. Briefly, mice were group housed and individually placed into well-ventilated 50 ml conical tubes for 2 h each day for 14 consecutive days. Body weight was measured in the morning of the first restraint session and again in the morning after the last restraint session.
Subchronic variable stress
Subchronic variable stress (SCVS) was performed as described previously [21]. Briefly, it consisted of three different stressors (foot shock, tail suspension and restraint stress) that were alternated over 6 days to avoid habituation. On day 1, foot shock consisted of 100 random mild electric shocks at 0.45 mA over 1 h. On day 2, tail suspension stress lasted 1 h. On day 3, restraint stress was applied by placing mice into 50 ml conical tubes for 1 h within their home cages. The same stressors were repeated for the next 3 days in the same order. Body weight was measured in the morning of the first stress session and again in the morning after the last stress session.
Forced swim test
Forced swim test (FST) was performed under bright light. Mice were placed in 4 L beakers containing ~3 L of tap water at a temperature of 25 ± 1 °C for 6 min. Behavior was recorded and immobility time, defined as the absence of any movement except that necessary for the mice to keep their heads above water, was manually counted over the last 4 min.
Open field test
The open field test (OFT) was performed under red light. Mice were placed in an open field arena (44 × 44 cm), and video tracking software (Ethovision 3.0, Noldus Information Technology, Leesburg, Virginia) was used to measure total movement of mice over 10 min.
Sucrose splash test
The sucrose splash test (SST) was performed as described previously [22]. Briefly, mice were sprayed with 10% sucrose solution onto their dorsal coat in the home cages under red light. After sucrose solution was applied, the behavior was recorded for 5 min. The time spent grooming was manually counted for the entire 5 min.
Female urine sniffing test
The female urine sniffing test (FUST) was performed as described previously [23] with minor modifications. Mice were first habituated to a cotton tip applied with sterile water for 1 h in the home cages under red light. Then the cotton tip was removed, and mice were left undisturbed for 45 min. Subsequently, a cotton tip applied with 75 μl of female urine was inserted into the cage and the behavior was recorded for 5 min. The time spent sniffing the cotton tip was manually counted for the entire 5 min.
qPCR and western blot analyses
cDNA from human postmortem tissue and mouse vmPFC was subjected to qPCR analysis using TaqMan universal PCR master mix (Thermo Fisher Scientific, Waltham, Massachusetts) and PerfeCta SYBR Green FastMix (Quanta Biosciences, Beverly, Massachusetts), respectively. Total homogenate and synaptosomes were analyzed by SDS-PAGE and western blotting [10]. Detailed descriptions are included in Supplemental Methods.
Drugs and treatments
Ketamine (20 mg/kg, i.p., Vedco, Saint Joseph, Missouri) was diluted in 0.9% saline. Xestospongin C (2.2 ng/side, intra-vmPFC, #ab120914, Abcam, Cambridge, Massachusetts) was dissolved in DMSO before diluting in 0.9% saline (0.5% final DMSO concentration). SKF96365 (0.2 μg/side, intra-vmPFC, #ab120280, Abcam, Cambridge, Massachusetts) was dissolved in 0.9% saline.
Human postmortem tissues
Demographic characteristics associated with the human tissue samples, provided by the Dallas Brain Collection and Quebec Suicide Brain Bank, are listed in Supplemental Methods and Supplemental Table 1.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 7. Details of statistical analyses including test used, exact sample sizes and P values for each figure are included in the Figure Legends, Supplemental Methods and/or in Supplemental Table 2.
Results
VGF expression is downregulated in BA25 in depressed patients and in the vmPFC in mice following chronic restraint stress (CRS)
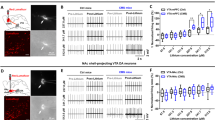
We determined VGF mRNA levels in human postmortem BA25 from MDD patients and healthy controls. VGF mRNA levels were significantly decreased in BA25 of both male and female (Fig. 1a) MDD patients compared to control subjects. To validate these human findings in mice, we exposed two cohorts of male wild-type mice to 14 days of CRS and then behaviorally assessed one cohort and collected vmPFC for gene expression analysis from another identically treated, independent cohort (Fig. 1b). Body weight measurements were conducted on both cohorts. We chose body weight measurement, sucrose splash test (SST) and forced swim test (FST) to determine depression-related phenotypes, as these assess body weight loss, lack of motivation, and coping strategy to acute stress, which recapitulate some of the core symptoms of human MDD patients [24, 25] and represent the process that is potentially affected by MDD [26]. Stressed mice showed significantly reduced body weight, decreased grooming time in the SST, and increased immobility time in the FST without significant difference in overall locomotor activity (Fig. 1c–f). To investigate whether VGF is regulated in the vmPFC by CRS, vmPFC was dissected 48 h after the last restraint session for western blot analysis (Fig. 1g). Levels of the VGF proprotein (detected as a ~90 kD doublet by antibody specific for VGF C-terminus) [27] were significantly reduced in the vmPFC of stressed mice compared to unstressed control mice (Fig. 1h). Similarly, in female wild-type mice that were exposed to subchronic variable stress (SCVS) (Supplemental Fig. 1A), VGF mRNA levels were reduced in the vmPFC (Supplemental Fig. 1B).
VGF expression is regulated by depression in the vmPFC. a Human VGF mRNA levels were reduced in postmortem Brodmann area (BA) 25 of male and female MDD subjects compared to controls (n = 15–16 per group for male, n = 11–13 per group for female). b Timeline of 14-day chronic restraint stress (CRS) experiment and behavioral tests on male mice. c Body weight was reduced in male mice subjected to CRS (n = 13–15 per group). Body weight data from all control and stressed mice are presented, including those sacrificed for VGF expression analysis without behavioral testing. d No significant difference in locomotor activity was observed between control and CRS-exposed male mice (n = 9 per group). e Grooming time in the SST was reduced in male mice subjected to CRS (n = 8–9 per group). f Immobility time in the FST was increased in male mice subjected to CRS (n = 8–9 per group). g Coronal schematics of mouse brain highlighting vmPFC collected (gray circle). Brain atlas adapted from Allen Brain Institute [67]. h VGF protein levels were reduced in vmPFC of male mice subjected to CRS compared to controls (n = 8–9 per group). All data are presented as mean ± s.e.m. Two-way analysis of variance following by Bonferroni’s multiple comparisons test (male) or Student’s t-test (female) for (a); Student’s t-test for (c, d–f, h); (t* P < 0.05 using Student’s t-test, *P < 0.05, ***P < 0.001, ****P < 0.0001). CTL control, CRS chronic restraint stress, BW body weight, OFT open field test, SST sucrose splash test, TC tissue collection, FST forced swim test
VGF overexpression in the vmPFC prevents the development of depression-related behaviors following CRS
Having shown that CRS exposure reduces VGF expression in the vmPFC and induces depression-related phenotypes, we investigated whether virus-mediated VGF overexpression in the vmPFC is sufficient to rescue CRS-induced depression-related phenotypes by injecting AAV5-VGF or AAV5-GFP into the vmPFC of wild-type mice. After 21 days of recovery, mice were subjected to CRS and subsequently assessed for behaviors (Fig. 2a). VGF overexpression was confirmed by western blot (Fig. 2b). Consistent with our above-mentioned findings, CRS reduced body weight, decreased grooming time in the SST and increased immobility time in the FST in AAV-GFP-injected controls but not in AAV-VGF-injected mice, without affecting overall locomotor activity (Fig. 2c–f). Western blot analysis revealed that VGF overexpression in the vmPFC alleviated the CRS-induced GluR1 reduction in synaptosomes 24 h after the last behavioral testing (Fig. 2g).
AAV-VGF-mediated VGF overexpression in vmPFC increases resilience to chronic restraint stress. a Timeline of stereotaxic surgery, chronic restraint stress (CRS) and behavioral tests. b Western blot analysis showed significantly increased VGF protein levels in vmPFC of AAV-VGF-injected wild-type mice sacrificed 21 days after virus injection (n = 4–5 per group). c AAV-VGF-injected wild-type mice showed increased body weight compared to AAV-GFP-injected wild-type mice following CRS (n = 8–9 per group). d No significant difference in locomotor activity was observed between vmPFC-AAV-GFP-injected and vmPFC-AAV-VGF-injected wild-type mice in the OFT (n = 7–9 per group). e AAV-VGF-injected wild-type mice showed increased grooming time in the SST compared to AAV-GFP-injected wild-type mice following CRS (n = 8–9 per group). f AAV-VGF-injected wild-type mice showed reduced immobility time in the FST compared to AAV-GFP-injected wild-type mice following CRS (n = 8–9 per group). g The total abundance of GluR1 was significantly reduced in synaptosomes of AAV-GFP- but not AAV-VGF-injected wild-type mice following CRS (n = 4–5 per group). All data are presented as mean ± s.e.m. Student’s t-test for b. Two-way analysis of variance following by Bonferroni’s multiple comparisons test or Student’s t-test for c–g (t* P < 0.05 using Student’s t-test, *P < 0.05, ***P < 0.001, ****P < 0.0001). CTL control, CRS chronic restraint stress, BW body weight, OFT open field test, SST sucrose splash test, FST forced swim test, TC tissue collection
VGF deficiency in the vmPFC increases susceptibility to subchronic variable stress (SCVS)
To determine the roles of vmPFC VGF in regulating depression-related behaviors, we stereotaxically administered adeno-associated virus (AAV), either AAV2-Cre or AAV2-GFP, into the vmPFC of homozygous floxed VGF (Vgfff/ff) mice [12] and behaviorally assessed them following SCVS exposure (Fig. 3a). SCVS is sufficient to induce depression-related phenotypes in female but not male mice [21], which makes it a useful tool to examine increased stress susceptibility in male mice. Localized Vgf ablation in the vmPFC was confirmed using western blot analysis (Fig. 3b). Consistent with a previous report in male wild-type mice, SCVS failed to induce depression-related phenotypes in AAV-GFP-injected male Vgfff/ff mice and did not affect overall locomotor activity (Fig. 3c–g). However, stressed AAV-Cre-injected Vgfff/ff mice showed significantly reduced body weight, decreased grooming time in the SST, and decreased sniffing time in the female urine sniffing test (FUST) compared to unstressed AAV-Cre-injected mice, and increased immobility time in the FST compared to stressed AAV-GFP-injected mice (Fig. 3c–g). To investigate the potential molecular mechanism underlying VGF knockdown-mediated susceptibility, we analyzed levels of the AMPA receptor subunit, GluR1, in synaptosomes of vmPFC 24 h after the last behavioral testing. We observed a significant reduction of GluR1 in SCVS-exposed AAV-Cre-injected mice compared to stressed AAV-GFP-injected mice, which is absent in control mice (Fig. 3h).
AAV-Cre-mediated VGF knockdown in vmPFC increases susceptibility to subthreshold stress. a Timeline of stereotaxic surgery, subchronic variable stress (SCVS) and behavioral tests. b Western blot analysis showed significantly reduced VGF protein levels in vmPFC of AAV-Cre-injected Vgfff/ff mice sacrificed 21 days after virus injection (n = 4–5 per group). c AAV-Cre-injected Vgfff/ff mice showed reduced body weight compared to AAV-GFP-injected Vgfff/ff mice following SCVS (n = 7–8 per group). d No significant difference in locomotor activity was observed between vmPFC-AAV-GFP- and vmPFC-AAV-Cre-injected Vgfff/ff mice in the OFT (n = 7–8 per group). e AAV-Cre-injected Vgfff/ff mice showed reduced grooming time in the SST compared to AAV-GFP-injected Vgfff/ff mice following SCVS (n = 7–8 per group). f AAV-Cre-injected Vgfff/ff mice showed reduced sniffing time in the FUST compared to AAV-GFP-injected Vgfff/ff mice following SCVS (n = 6–8 per group). g AAV-Cre-injected Vgfff/ff mice showed increased immobility time in the FST compared to AAV-GFP-injected Vgfff/ff mice following SCVS (n = 7–8 per group). h The total abundance of GluR1 was significantly reduced in synaptosomes of AAV-Cre-injected but not AAV-GFP-injected Vgfff/ff mice following SCVS (n = 4–5 per group). All data are presented as mean ± s.e.m. Student’s t-test for b. Two-way analysis of variance following by Bonferroni’s multiple comparisons test or Student’s t-test for c–h (t* P < 0.05 using Student’s t-test, *P < 0.05, **P < 0.01, ***P < 0.001, **** P < 0.0001). CTL control, SCVS subchronic variable stress, BW body weight, OFT open field test, SST sucrose splash test, FUST female urine sniffing test, FST forced swim test, TC tissue collection
VGF knockdown in the vmPFC blocks the antidepressant effects of ketamine
Previous studies reported that VGF expression is upregulated in hippocampus by exercise, and by imipramine or ketamine treatment [9,10,11]. Here, we examined VGF expression in the vmPFC at various time points after acute ketamine injection (20 mg/kg, i.p.) (Fig. 4a). This dose has been previously reported to induce antidepressant effects in rodent models [10, 28]. VGF protein levels were significantly upregulated by ketamine in the total homogenate of the vmPFC at 30 min, 24 h, 3 days and 7 days post treatment compared to their respective controls (Fig. 4b). To test whether VGF expression in the vmPFC is required for the rapid antidepressant effects of ketamine, we subjected AAV-Cre/AAV-GFP-injected Vgfff/ff mice to the FST or OFT 30 min after ketamine treatment. We found that VGF knockdown in the vmPFC abolished ketamine-induced reduction in immobility time at this time point (Fig. 4c) without affecting locomotor activity (Fig. 4d). In addition, we determined the requirement of vmPFC VGF in sustained action of ketamine by subjecting AAV-Cre/AAV-GFP-injected Vgfff/ff mice to behavioral assessments starting at the 3rd day after ketamine treatment (Fig. 4e). Ketamine did not affect locomotor activity on the 3rd day after treatment (Fig. 4f), and its antidepressant effects were evident in the SST and FST on the 5th and 7th days after treatment, respectively (Fig. 4g, h). Western blot analysis showed that ketamine treatment increases GluR1 level in vmPFC synaptosomes in AAV-GFP-injected mice on the 8th day after injection, which was absent in AAV-Cre-injected mice (Fig. 4i).
VGF deficiency in vmPFC attenuates the efficacy of the rapid-acting antidepressant ketamine. a Timeline of acute ketamine treatment (20 mg/kg, i.p.) and tissue collection time points. b Acute ketamine treatment (20 mg/kg, i.p.) significantly increased VGF protein expression in the vmPFC 30 min, 24 h, 3 days and 7 days post injection (30 min: n = 4 per group; 24 h: n = 5–6 per group; 3 d: n = 4 per group; 7 d: n = 5 per group). (c) Acute ketamine treatment (20 mg/kg, i.p.) significantly reduced the immobility time in the FST in vmPFC-AAV-GFP- but not AAV-Cre-injected Vgfff/ff mice, compared to saline treatment 30 min post injection (n = 9–10 per group). d Acute ketamine treatment (20 mg/kg, i.p.) did not affect the locomotor activity in vmPFC-AAV-GFP-injected and AAV-Cre-injected Vgfff/ff mice 30 min post injection (n = 10–11 per group). e Timeline of acute ketamine treatment paradigm and behavioral tests. f Acute ketamine treatment (20 mg/kg, i.p.) did not affect the locomotor activity in vmPFC-AAV-GFP-injected and AAV-Cre-injected Vgfff/ff mice on the 3rd day post injection (n = 5–6 per group). g Acute ketamine treatment (20 mg/kg, i.p.) significantly increased grooming time in the SST in vmPFC-AAV-GFP- but not AAV-Cre-injected Vgfff/ff mice compared to saline treatment on the 5th day post injection (n = 5–6 per group). h Acute ketamine treatment (20 mg/kg, i.p.) significantly reduced immobility time in the FST in vmPFC-AAV-GFP-injected but not AAV-Cre-injected Vgfff/ff mice compared to saline treatment on the 7th day post injection (n = 5–6 per group). i The total abundance of GluR1 was significantly increased in synaptosomes of AAV-GFP-injected but not AAV-Cre-injected Vgfff/ff mice receiving ketamine (n = 5 per group). All data are presented as mean ± s.e.m. Student’s t-test for b. Two-way analysis of variance following by Bonferroni’s multiple comparisons test for (c, d, f–i) or Student’s t-test (g) (t* P < 0.05 using Student's t-test, *P < 0.05). OFT open field test, SST sucrose splash test, FST forced swim test, TC tissue collection, SAL saline, KET ketamine
Acute intra-vmPFC infusion of VGF C-terminal peptide TLQP-62 produces sustained antidepressant-like effects that require vmPFC BDNF expression and calcium mobilization
Previous studies demonstrated that acute intra-hippocampal infusion of TLQP-62 induces both rapid and sustained antidepressant-like effects [9, 10], with rapid efficacy requiring hippocampal BDNF expression [10]. We therefore investigated whether intra-vmPFC TLQP-62 (0.5 µg/side) infusion induced sustained behavioral phenotypes that mimic antidepressant drug treatment-induced behaviors, and whether this was dependent on vmPFC BDNF expression. Our results demonstrate that intra-vmPFC TLQP-62 infusion produced sustained antidepressant-like effects that last up to 7 days after treatment, which were blocked by AAV-Cre-mediated BDNF ablation in the vmPFC of homozygous Bdnfflox/flox mice (Fig. 5a–d), suggesting that vmPFC BDNF is required for the sustained actions of TLQP-62. Notably, the antidepressant actions of ketamine, scopolamine and GLYX-13, all of which exhibit comparable rapid and sustained effects as seen with TLQP-62, have been demonstrated to be dependent on BDNF [29,30,31].
The antidepressant efficacy of acute intra-vmPFC TLQP-62 infusion requires vmPFC BDNF and Ca2+ mobilization. a Timeline of behavioral paradigm for vmPFC AAV-Cre-injected Bdnfflox/flox mice receiving TLQP-62 infusion. b No significant difference was observed in locomotor activity between vmPFC AAV-GFP-injected and AAV-Cre-injected Bdnfflox/flox mice on the 3rd day post TLQP-62 (0.5 μg/side) infusion (n = 8–9 per group). c vmPFC AAV-GFP-, but not AAV-Cre-injected Bdnfflox/flox mice displayed increased grooming time in the SST on the 5th day post TLQP-62 (0.5 μg/side) infusion (n = 8–9 per group). d vmPFC AAV-GFP-, but not AAV-Cre injected Bdnfflox/flox mice displayed reduced immobility time in the FST on the 7th day post TLQP-62 (0.5 μg/side) infusion (n = 8–9 per group). e The total abundance of GluR1 was significantly increased in synaptosomes of AAV-GFP-injected but not AAV-Cre-injected Bdnfflox/flox mice receiving TLQP-62 (n = 5–6 per group). f Timeline of behavioral paradigm for wild-type mice receiving TLQP-62 infusion following pretreatment with xestospongin C (XeC). g No significant difference was observed in locomotor activity between XeC and DMSO pretreated wild-type mice on the 3rd day post TLQP-62 (0.5 μg/side) infusion (n = 6–7 per group). h Wild-type mice pretreated with DMSO but not XeC displayed increased grooming time in the SST on the 5th day post TLQP-62 (0.5 μg/side) infusion (n = 6–7 per group). i Wild-type mice pretreated with DMSO but not XeC displayed reduced immobility time in the FST on the 7th day post TLQP-62 (0.5 μg/side) infusion (n = 6–7 per group). j The total abundance of GluR1 was significantly increased in synaptosomes of DMSO- but not XeC-pretreated wild-type mice receiving TLQP-62 (n = 5 per group). k Timeline of behavioral paradigm for wild-type mice receiving TLQP-62 infusion following pretreatment with SKF96365 (SKF). l No significant difference was observed in locomotor activity between SKF and saline pretreated wild-type mice on the 3rd day post TLQP-62 (0.5 μg/side) infusion (n = 7 per group). m Wild-type mice pretreated with saline but not SKF displayed increased grooming time in the SST on the 5th day post TLQP-62 (0.5 μg/side) infusion (n = 7 per group). n Wild-type mice pretreated with saline but not SKF displayed reduced immobility time in the FST on the 7th day post TLQP-62 (0.5 μg/side) infusion (n = 7 per group). o The total abundance of GluR1 was significantly increased in synaptosomes of saline- but not SKF-pretreated wild-type mice receiving TLQP-62 (n = 6 per group). p Schematic model of antidepressant actions of TLQP-62. We propose that TLQP-62 induces IP3-mediated Ca2+ release from endoplasmic reticulum (ER), depleting Ca2+ pool in ER, which is sensed by stromal interaction molecule 1 (STIM1). STIM1 then activates Ca2+ channel on the membrane, which is formed by Orai or TRPC, allowing Ca2+ influx via store-operated calcium entry (SOCE) and/or TRPC-mediated extracellular calcium entry. The increased intracellular Ca2+ level stimulates BDNF secretion, promotes BDNF/TrkB signaling and the mTOR pathway, then rapid local synthesis and secretion of VGF (TLQP-62) and BDNF, and synthesis, phosphorylation and insertion of GluR1. All data are presented as mean ± s.e.m. Two-way analysis of variance following by Bonferroni’s multiple comparisons test for b–e, g–j, l–o (#P < 0.10, *P < 0.05, **P < 0.01, ***P < 0.001). OFT open field test, SST sucrose splash test, FST forced swim test, TC tissue collection, STIM1 stromal interaction molecule 1, Orai calcium-release-activated calcium channel protein, TRPC transient receptor potential canonical, XeC xestospongin C, SKF SKF96365
TLQP-62 has been reported to induce insulin secretion in insulinoma cells by increasing intracellular calcium mobilization [32], which is one of the cellular mechanisms underlying BDNF secretion in neurons [33]. To test whether the sustained antidepressant-like efficacy of intra-vmPFC TLQP-62 infusion depends on intracellular calcium mobilization, we locally pretreated the mice in the vmPFC with xestospongin C (2.2 ng/side), an inhibitor of inositol 1,4,5-trisphosphate (IP3)-dependent calcium release [34], 25 min before peptide infusion (Fig. 5f). Subsequent behavioral tests indicated that xestospongin C pretreatment did not affect the behaviors of SC-62-infused mice in the OFT, SST and FST, but did block the behavioral responses in TLQP-62-infused mice (Fig. 5g–i). Store-operated calcium entry (SOCE) via Orai and/or TRPC channels is a Ca2+ entry mechanism that is activated following IP3-mediated Ca2+ release from the endoplasmic reticulum (ER) and consequent reduction of luminal ER Ca2+ [35]. To test whether SOCE is involved in the sustained actions of intra-vmPFC TLQP-62 infusion, we locally pretreated mice in the vmPFC with SKF96365 (0.2 µg/side), a SOCE inhibitor [36], 25 min before peptide infusion (Fig. 5k). We found that SKF96365 pretreatment completely blocked the behavioral responses to TLQP-62 infusion (Fig. 5l–n). In addition, on the 8th day after TLQP-62 infusion, we collected the vmPFC and analyzed GluR1 levels in synaptosomes. We found that TLQP-62 infusion significantly increased GluR1 abundance in vmPFC synaptosomes, which was blocked by BDNF knockdown, as well as by pretreatment with xestospongin C or SKF96365 (Fig. 5e, j, o). These data suggest that increased synaptic expression of GluR1, regulated by IP3-mediated Ca2+ release, SOCE, and/or TRPC-mediated extracellular calcium entry, could contribute to the molecular mechanisms that underlie the antidepressant actions of intra-vmPFC TLQP-62 infusion (Fig. 5p).
Discussion
In the current study, we demonstrated that VGF mRNA expression is reduced in postmortem BA25 of human MDD subjects. BA25 is the posterior portion of sgACC [3], which is importantly implicated in the pathophysiology of MDD [4]. Our observation that VGF is specifically reduced in BA25 complements a previous study documenting reduced VGF broadly in sgACC [18]. Reduced levels of inhibitory interneuron markers have been reported in sgACC of MDD subjects [18], which is consistent with reduced density of inhibitory interneurons observed in the prefrontal cortex in this condition [37]. VGF is expressed in interneurons [38], and it has been proposed that VGF synthesized in and secreted from inhibitory interneurons may play an important role in regulating depression-related behaviors [10]. Thus, lower VGF levels in BA25 of MDD patients could possibly result from reduced inhibitory interneuron numbers. Reduced volume has been documented in sgACC of MDD subjects [4], which is attributed to decreased number and density of glial cells [39], likely oligodendrocytes [40]. Because VGF promotes oligodendrogenesis and myelination [41], reduced VGF levels in BA25 could potentially lead to a reduction in the number of oligodendrocytes, and hence contribute to volumetric shrinkage in this region.
One limitation of our current study is that there are differences in PMI between control and MDD in our limited cohort of human postmortem samples (Supplemental Table 1). However, there is no significant correlation between PMI and VGF mRNA levels in both male and female subjects (Supplemental Fig. 2A, B). Indeed, our findings that VGF expression is downregulated in the vmPFC (closely matched in PMI) of male mice exposed to CRS and female mice exposed to SCVS lend support to the validity of our human postmortem study. The rodent vmPFC correlates to sgACC of humans, as the infralimbic cortex (IL-PFC; a portion of rodent vmPFC) anatomically correlates to human BA25 region [42]. Rodent vmPFC has been extensively implicated in depression-related behaviors and response to antidepressant treatment. Chronic social defeat stress reduced neuronal activity in the vmPFC [6], while optogenetic stimulation of IL-PFC has been shown to induce antidepressant effects [6] and silencing of IL-PFC neurons is sufficient to block the antidepressant actions of ketamine [43]. Our findings in the vmPFC, together with our recent study demonstrating that hippocampal VGF is downregulated in stressed mice [10], are consistent with previous reports that documented reduced BDNF levels in the PFC and hippocampus in stressed rodents [44], suggesting that VGF, within the prefrontal cortical-limbic circuit, plays an important role in regulating depression-related behavior, as does BDNF. Connectivity between hippocampus and vmPFC is sensitive to stress [45]. Thus parallel, stress-induced changes in VGF expression in the vmPFC and hippocampus may significantly contribute to the associated changes in volume and dendritic morphology that are observed in these two brain regions in stressed animals [46], and potentially could affect the connectivity between these regions as VGF C-terminal peptides TLQP-62 and AQEE-30, have been shown to potentiate synaptic transmission (21), promote dendritic maturation [47], and induce synaptogenesis (20). That ablation of VGF in either vmPFC (shown here) or dHc (shown previously in refs [10, 48]) each increased sensitivity to stress or blocked rapid-acting antidepressant efficacy, is also suggestive that neural pathways connecting vmPFC and dHc, which could be impacted by either dHc or vmPFC manipulation, are critical to the actions of VGF in the regulation of depression-related behaviors.
Importantly, our study revealed that VGF levels in the vmPFC dictate susceptibility or resilience to stress exposure. VGF deficiency in vmPFC led to increased susceptibility to subthreshold stress exposure in SCVS. Previous studies suggest that vmPFC dysfunction could result in abnormal stress responses [49]. VGF plays an indispensable role in the formation of secretory granules [50, 51], the regulated release of neurotrophins and other growth factors [13], and the promotion of neuronal survival and dendritic outgrowth [47, 52]. Reduced VGF levels could perhaps dysregulate neuronal homeostasis in the vmPFC, disrupting its normal function and increasing susceptibility to stress. Importantly, VGF knockdown in vmPFC in unstressed control mice did not affect their behaviors, indicating that VGF-mediated effects on susceptibility require stress exposure. Conversely, VGF overexpression in vmPFC promoted resilience to chronic stress exposure in CRS, perhaps by preventing the downregulation of VGF expression in vmPFC that is caused by CRS, thus maintaining normal vmPFC function in response to stress exposure. Previous studies have shown that GluR1 knockout mice exhibit depression-related behaviors [17] and that GluR1 is downregulated in the PFC [16] following chronic stress exposure. Our observations that VGF knockdown results in lower synaptosomal GluR1 levels following subthreshold stress exposure and that VGF overexpression abolishes chronic stress-induced GluR1 reduction indicate that VGF-dependent changes in GluR1 levels following stress exposure likely contribute to the development of susceptibility or resilience.
In the present study, we report that acute ketamine treatment increases VGF protein levels in the vmPFC as early as 30 min and for up to 7 days post injection. This rapid increase of VGF protein is consistent with our previously observed VGF induction in hippocampus [10] and comparable to reported BDNF elevations in hippocampus [53]. A sustained increase of VGF protein levels is in line with long-lasting BDNF induction previously demonstrated in both PFC and hippocampus [54]. We also showed that VGF knockdown in the vmPFC blocks both rapid and sustained antidepressant effects of ketamine, suggesting that VGF expression in the vmPFC is required for the actions of ketamine, as it is in hippocampus [10]. Phosphorylation of GluR1 Ser845 regulates trafficking and incorporation of GluR1-containing AMPA receptors into synapses [55]. It has been shown that ketamine rapidly induces GluR1 phosphorylation at Ser845 in hippocampus in a VGF-dependent manner [10], leading to increased GluR1 levels in synaptosomes, and that GluR1 Ser845 phosphorylation is required for both rapid and sustained antidepressant actions of ketamine [56]. We documented ketamine-induced GluR1 elevation in synaptosomes that lasts up to 8 days post treatment, in line with a previous report [57]. That VGF knockdown abolishes this GluR1 elevation and ketamine’s antidepressant action indicates that VGF-mediated synaptic incorporation of GluR1-containing AMPA receptors and subsequent synaptic potentiation [58] may underlie the sustained antidepressant actions of ketamine.
Finally, we confirmed that a single acute intra-vmPFC infusion of VGF C-terminal peptide TLQP-62 produces sustained (up to 7 days) behavioral changes that mimic antidepressant drug treatment-induced behaviors, which complements our previous study demonstrating rapid antidepressant effects of intra-hippocampal TLQP-62 infusion 30 min after administration [10]. The sustained actions of intra-vmPFC TLQP-62 infusion also parallel the previously demonstrated time frame of ketamine’s antidepressant efficacy [57] that was reproduced in this study, and sustained efficacy of TLQP-62 previously noted 6 days after infusion into mouse hippocampus [9]. Previous studies reported that reduced hippocampal BDNF expression blocks the antidepressant effects of intra-hippocampal TLQP-62 infusion [10] and ketamine treatment [53]. Similarly, reduced vmPFC BDNF expression blocks the sustained antidepressant actions of intra-vmPFC infused TLQP-62. BDNFMet/Met mice, which exhibit disrupted BDNF distribution, sorting, trafficking and secretion [59], show impaired responses to novel antidepressants such as ketamine, scopolamine and GLYX-13 [29,30,31]. Could the antidepressant actions of intra-vmPFC TLQP-62 also be dependent on BDNF trafficking and secretion in this region? Previous studies strongly suggest that TLQP-62 may be a critical component of a positive autoregulatory feedback loop that potentiates local BDNF synthesis and secretion and BDNF/TrkB signaling [60], regulating depression-related behaviors and memory. Moreover, we found that local BDNF knockdown abolishes increased GluR1 levels in vmPFC synaptosomes 8 days after TLQP-62 infusion. This observation complements our previous report that local BDNF expression is necessary for increased GluR1 phosphorylation 2 h after intra-hippocampal TLQP-62 administration [10], indicating that normal BDNF levels are required for TLQP-62-induced synaptic trafficking of GluR1 and synaptic incorporation of GluR1-containing AMPARs.
The importance of Ca2+ signaling in depression and the response to antidepressant treatment has been extensively demonstrated [29,30,31, 61]. Here, we showed that the antidepressant effects and increased GluR1 levels in synaptosomes induced by intra-vmPFC TLQP-62 infusion are blocked by local pretreatment with xestospongin C or SKF96365, which inhibit IP3-mediated Ca2+ release from endoplasmic reticulum (ER) [34] and SOCE via Orai and TRPC channels [36, 62], respectively. Ca2+ store depletion from ER caused by Ca2+ release induces conformational transition of STIM1, an ER Ca2+ content sensor, which enables STIM1 to interact with and activate Orai and TRPC channels to allow Ca2+ influx (SOCE) [63]. Indeed, evidence supports requirements for ER-dependent Ca2+ release and SOCE in BDNF secretion [33, 64] and synaptic GluR1 trafficking [65], both of which have been proposed to be mechanisms underlying the antidepressant actions of intra-hippocampal TLQP-62 administration [10]. Consistent with our observation that intracellular Ca2+ mobilization and SOCE is necessary for TLQP-62-induced GluR1 elevation in vmPFC synaptosomes, BDNF has been reported to regulate GluR1 trafficking via IP3- and SOCE-mediated Ca2+ signaling [65]. TRPC channels can also function in a STIM1-independent manner (SOCE-independent) [63], and the calcium channel blocker SKF96365 notably has broad, dose-dependent activity against TRPC (SOCE or receptor-mediated calcium entry), T-type, and L-type channels [66]. Because the TLQP-62 receptor also remains to be identified, further studies are warranted to more precisely delineate the cellular mechanisms underlying TLQP-62-induced Ca2+ influx.
In conclusion, our results suggest that vmPFC VGF is critically involved in the pathophysiology of depression. We propose that VGF and its C-terminal peptide TLQP-62 in the vmPFC potentially increase intracellular Ca2+ levels via IP3-mediated Ca2+ release from ER, and/or via Ca2+ influx mediated by SOCE and/or TRPC mechanisms, which subsequently stimulate BDNF secretion. This promotes BDNF/TrkB signaling and mTOR pathway activation, resulting in increased expression and phosphorylation (Ser845) of GluR1, stimulating synaptic trafficking of GluR1 and GluR1-dependent synaptic potentiation [58], thus regulating depression-related behaviors and the response to ketamine [10, 60].
References
Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005;62:593.
Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–43.
Johansen-Berg H, Gutman DA, Behrens TEJ, Matthews PM, Rushworth MFS, Katz E, et al. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18:1374–83.
Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–81.
Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 2009;33:773–83.
Covington HE, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, et al. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J Neurosci. 2010;30:16082–90.
Chang CH, Chen MC, Qiu MH, Lu J. Ventromedial prefrontal cortex regulates depressive-like behavior and rapid eye movement sleep in the rat. Neuropharmacology. 2014;86:125–32.
Jiang C, Salton S. The role of neurotrophins in major depressive disorder. Transl Neurosci. 2013;4:46–58.
Thakker-Varia S, Krol JJ, Nettleton J, Bilimoria PM, Bangasser DA, Shors TJ, et al. The neuropeptide VGF produces antidepressant-like behavioral effects and enhances proliferation in the hippocampus. J Neurosci. 2007;27:12156–67.
Jiang C, Lin W-J, Sadahiro M, Labonté B, Menard C, Pfau ML, et al. VGF function in depression and antidepressant efficacy. Mol Psychiatry. 2018;23:1632–42.
Hunsberger JG, Newton SS, Bennett AH, Duman CH, Russell DS, Salton SR, et al. Antidepressant actions of the exercise-regulated gene VGF. Nat Med. 2007;13:1476–82.
Lin W-J, Jiang C, Sadahiro M, Bozdagi O, Vulchanova L, Alberini CM, et al. VGF and Its C-Terminal Peptide TLQP-62 Regulate Memory Formation in Hippocampus via a BDNF-TrkB-Dependent Mechanism. J Neurosci. 2015;35:10343–56.
Bozdagi O, Rich E, Tronel S, Sadahiro M, Patterson K, Shapiro ML, et al. The neurotrophin-inducible gene Vgf regulates hippocampal function and behavior through a brain-derived neurotrophic factor-dependent mechanism. J Neurosci. 2008;28:9857–69.
Alder J, Thakker-Varia S, Bangasser DA, Kuroiwa M, Plummer MR, Shors TJ, et al. Brain-derived neurotrophic factor-induced gene expression reveals novel actions of VGF in hippocampal synaptic plasticity. J Neurosci. 2003;23:10800–8.
Thakker-Varia S, Behnke J, Doobin D, Dalal V, Thakkar K, Khadim F, et al. VGF (TLQP-62)-induced neurogenesis targets early phase neural progenitor cells in the adult hippocampus and requires glutamate and BDNF signaling. Stem Cell Res. 2014;12:762–77.
Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated Stress Causes Cognitive Impairment by Suppressing Glutamate Receptor Expression and Function in Prefrontal Cortex. Neuron. 2011;73:962–77.
Chourbaji S, Vogt MA, Fumagalli F, Sohr R, Frasca A, Brandwein C, et al. AMPA receptor subunit 1 (GluR-A) knockout mice model the glutamate hypothesis of depression. FASEB J. 2008;22:3129–34.
Tripp A, Oh H, Guilloux J-P, Martinowich K, Lewis DA, Sibille E. Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am J Psychiatry. 2012;169:1194–202.
Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–57.
Seo J-S, Wei J, Qin L, Kim Y, Yan Z, Greengard P. Cellular and molecular basis for stress-induced depression. Mol Psychiatry. 2017;22:1440–7.
Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, et al. Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J Neurosci. 2015;35:16362–76.
Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–9.
Malkesman O, Scattoni ML, Paredes D, Tragon T, Pearson B, Shaltiel G, et al. The female urine sniffing test: a novel approach for assessing reward-seeking behavior in rodents. Biol Psychiatry. 2010;67:864–71.
Nestler EJ, Gould E, Manji H. Preclinical models: status of basic research in depression. Biol Psychiatry. 2002;52:503–28.
Isingrini E, Camus V, Le Guisquet A-M, Pingaud M, Devers S, Belzung C. Association between repeated unpredictable chronic mild stress (UCMS) procedures with a high fat diet: a model of fluoxetine resistance in mice. PLoS One. 2010;5:e10404.
Commons KG, Cholanians AB, Babb JA, Ehlinger DG. The rodent forced swim test measures stress-coping strategy, not depression-like behavior. ACS Chem Neurosci. 2017; https://doi.org/10.1021/acschemneuro.7b00042.
Trani E, Giorgi A, Canu N, Amadoro G, Rinaldi AM, Halban PA, et al. Isolation and characterization of VGF peptides in rat brain. Role of PC1/3 and PC2 in the maturation of VGF precursor. J Neurochem. 2002;81:565–74.
Donahue RJ, Muschamp JW, Russo SJ, Nestler EJ, Carlezon WA. Effects of striatal ΔFosB overexpression and ketamine on social defeat stress–induced anhedonia in mice. Biol Psychiatry. 2014;76:550–558.
Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol. 2015;18. https://doi.org/10.1093/ijnp/pyu033.
Ghosal S, Bang E, Yue W, Hare BD, Lepack AE, Girgenti MJ, et al. Activity-dependent brain-derived neurotrophic factor release is required for the rapid antidepressant actions of scopolamine. Biol Psychiatry. 2018;83:29–37.
Kato T, Fogaça M V, Deyama S, Li X-Y, Fukumoto K, Duman RS. BDNF release and signaling are required for the antidepressant actions of GLYX-13. Mol Psychiatry. 2017. https://doi.org/10.1038/mp.2017.220.
Petrocchi-Passeri P, Cero C, Cutarelli A, Frank C, Severini C, Bartolomucci A, et al. The VGF-derived peptide TLQP-62 modulates insulin secretion and glucose homeostasis. J Mol Endocrinol. 2015;54:227–39.
Balkowiec A, Katz DM. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J Neurosci. 2002;22:10399–407.
Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, Molinski TF, et al. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron. 1997;19:723–33.
Prakriya M, Lewis RS. Store-operated calcium channels. Physiol Rev. 2015;95:1383–436.
Majewski L, Kuznicki J. SOCE in neurons: signaling or just refilling? Biochim Biophys Acta. 2015;1853:1940–52.
Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16:383–406.
Van Den Pol AN, Bina K, Decavel C, Ghosh P. VGF expression in the brain. J Comp Neurol. 1994;347:455–69.
Ongür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95:13290–5.
Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67:269–75.
Alvarez-Saavedra M, De Repentigny Y, Yang D, O’Meara RW, Yan K, Hashem LE, et al. Voluntary Running Triggers VGF-Mediated Oligodendrogenesis to Prolong the Lifespan of Snf2h-Null Ataxic Mice. Cell Rep. 2016;17:862–75.
Gabbott PLA, Warner TA, Jays PRL, Bacon SJ. Areal and synaptic interconnectivity of prelimbic (area 32), infralimbic (area 25) and insular cortices in the rat. Brain Res. 2003;993:59–71.
Fuchikami M, Thomas A, Liu R, Wohleb ES, Land BB, DiLeone RJ, et al. Optogenetic stimulation of infralimbic PFC reproduces ketamine’s rapid and sustained antidepressant actions. Proc Natl Acad Sci USA. 2015;112:8106–11.
Burton CL, Chatterjee D, Chatterjee-Chakraborty M, Lovic V, Grella SL, Steiner M, et al. Prenatal restraint stress and motherless rearing disrupts expression of plasticity markers and stress-induced corticosterone release in adult female Sprague–Dawley rats. Brain Res. 2007;1158:28–38.
Godsil BP, Kiss JP, Spedding M, Jay TM. The hippocampal–prefrontal pathway: the weak link in psychiatric disorders? Eur Neuropsychopharmacol. 2013;23:1165–81.
Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, et al. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry. 2009;14:764–73.
Behnke o, Cheedalla A, Bhatt V, Bhat M, Teng S, Palmieri A, et al. Neuropeptide VGF promotes maturation of hippocampal dendrites that is reduced by single nucleotide polymorphisms. Int J Mol Sci. 2017;18. https://doi.org/10.3390/ijms18030612.
Lu Y, Wang C, Xue Z, Li C, Zhang J, Zhao X, et al. PI3K/AKT/mTOR signaling-mediated neuropeptide VGF in the hippocampus of mice is involved in the rapid onset antidepressant-like effects of GLYX-13. Int J Neuropsychopharmacol. 2015;18. https://doi.org/10.1093/ijnp/pyu110.
Wang M, Perova Z, Arenkiel BR, Li B. Synaptic modifications in the medial prefrontal cortex in susceptibility and resilience to stress. J Neurosci. 2014;34:7485–92.
Fargali S, Garcia AL, Sadahiro M, Jiang C, Janssen WG, Lin W-J, et al. The granin VGF promotes genesis of secretory vesicles, and regulates circulating catecholamine levels and blood pressure. FASEB J. 2014;28:2120–33.
Stephens SB, Edwards RJ, Sadahiro M, Lin W-J, Jiang C, Salton SR, et al. The prohormone VGF regulates β cell function via insulin secretory granule biogenesis. Cell Rep. 2017;20:2480–9.
Sato H, Fukutani Y, Yamamoto Y, Tatara E, Takemoto M, Shimamura K, et al. Thalamus-derived molecules promote survival and dendritic growth of developing cortical neurons. J Neurosci. 2012;32:15388–402.
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng P, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature . 2011;475:91–5.
Dong C, Zhang J-C, Yao W, Ren Q, Ma M, Yang C, et al. Rapid and sustained antidepressant action of the mGlu2/3 receptor antagonist MGS0039 in the social defeat stress model: comparison with ketamine. Int J Neuropsychopharmacol. 2017;20:228–36.
Esteban JA, Shi S-H, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–43.
Zhang K, Xu T, Yuan Z, Wei Z, Yamaki VN, Huang M, et al. Essential roles of AMPA receptor GluA1 phosphorylation and presynaptic HCN channels in fast-acting antidepressant responses of ketamine. Sci Signal. 2016;9:ra123.
Li N, Liu R-J, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–61.
Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281:752–8.
Chen Z-Y, Jing D, Bath KG, Ieraci A, Khan T, Siao C-J, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–3.
Jiang C, Lin W-J, Salton SR. Role of a VGF/BDNF/TrkB autoregulatory feedback loop in rapid-acting antidepressant efficacy. J Mol Neurosci. 2018. https://doi.org/10.1007/s12031-018-1124-0. [Epub ahead of print]
Hullett FJ, Potkin SG, Levy AB, Ciasca R. Depression associated with nifedipine-induced calcium channel blockade. Am J Psychiatry. 1988;145:1277–9.
Liou J, Kim ML, Do Heo W, Jones JT, Myers JW, Ferrell JE, et al. STIM Is a Ca2+Sensor Essential for Ca2+-Store-Depletion-Triggered Ca2+Influx. Curr Biol. 2005;15:1235–41.
Choi S, Maleth J, Jha A, Lee KP, Kim MS, So I, et al. The TRPCs–STIM1–orai interaction. Cham: Springer; 2014. p. 1035–54.
Vohra PK, Thompson MA, Sathish V, Kiel A, Jerde C, Pabelick CM, et al. TRPC3 regulates release of brain-derived neurotrophic factor from human airway smooth muscle. Biochim Biophys Acta. 1833;2013:2953–60.
Nakata H, Nakamura S. Brain-derived neurotrophic factor regulates AMPA receptor trafficking to post-synaptic densities via IP3R and TRPC calcium signaling. FEBS Lett. 2007;581:2047–54.
Singh A, Hildebrand ME, Garcia E, Snutch TP. The transient receptor potential channel antagonist SKF96365 is a potent blocker of low-voltage-activated T-type calcium channels. Br J Pharmacol. 2010;160:1464–75.
Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–76.
FUNDING AND DISCLOSURE
Supported in part by NIH grants MH086499 (SRS), MH083496 (SRS), pilot grant on P50AT008661 (SRS; PI G.M. Pasinetti); Hope for Depression Research Foundation (SRS); Brain and Behavior Research Foundation (SRS); BrightFocus Foundation (SRS). The authors report no biomedical financial interests or potential conflicts of interest.
Authors contributions
CJ, SJR, and SRS designed research; CJ performed research; CJ analyzed data; BL, WJL, EJN, CAT, and GT provided reagents/materials; CJ and SRS wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Jiang, C., Lin, WJ., Labonté, B. et al. VGF and its C-terminal peptide TLQP-62 in ventromedial prefrontal cortex regulate depression-related behaviors and the response to ketamine. Neuropsychopharmacol. 44, 971–981 (2019). https://doi.org/10.1038/s41386-018-0277-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-018-0277-4
This article is cited by
-
The endogenous opioid system in the medial prefrontal cortex mediates ketamine’s antidepressant-like actions
Translational Psychiatry (2024)
-
Predicting the diagnosis of various mental disorders in a mixed cohort using blood-based multi-protein model: a machine learning approach
European Archives of Psychiatry and Clinical Neuroscience (2023)
-
A translational perspective on the anti-anhedonic effect of ketamine and its neural underpinnings
Molecular Psychiatry (2022)
-
Plexin-A1 expression in the inhibitory neurons of infralimbic cortex regulates the specificity of fear memory in male mice
Neuropsychopharmacology (2022)
-
Mechanisms of synaptic transmission dysregulation in the prefrontal cortex: pathophysiological implications
Molecular Psychiatry (2022)