Abstract
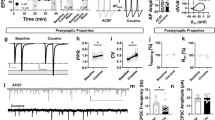
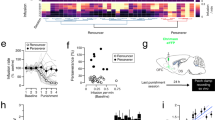
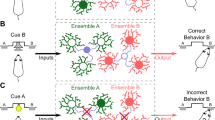
Both clinical and animal studies showed that the impaired functions of the orbitofrontal cortex (OFC) underlie the compulsive drug-seeking behavior of drug addiction. However, the functional changes of the microcircuit in the OFC and the underlying molecular mechanisms in drug addiction remain elusive, and little is known for whether microcircuits in the OFC contributed to drug addiction-related behaviors. Utilizing the cocaine-induced conditioned-place preference model, we found that the malfunction of the microcircuit led to disinhibition in the OFC after cocaine withdrawal. We further showed that enhanced Somatostatin-Parvalbumin (SST-PV) inhibitory synapse strength changed microcircuit function, and SST and PV inhibitory neurons showed opposite contributions to the drug addiction-related behavior of mice. Brevican of the perineuronal nets of PV neurons regulated SST-PV synapse strength, and the knockdown of Brevican alleviated cocaine preference. These results reveal a novel molecular mechanism of the regulation of microcircuit function and a novel circuit mechanism of the OFC in gating cocaine preference.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data are available upon request.
References
O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102.
Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, et al. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–42.
Bariselli S, Miyazaki NL, Creed MC, Kravitz AV. Orbitofrontal-striatal potentiation underlies cocaine-induced hyperactivity. Nat Commun. 2020;11:3996.
Harada M, Pascoli V, Hiver A, Flakowski J, Luscher C. Corticostriatal activity driving compulsive reward seeking. Biol Psychiatry. 2021;90:808–18.
Xue AM, Foerde K, Walsh BT, Steinglass JE, Shohamy D, Bakkour A. Neural representations of food-related attributes in the human orbitofrontal cortex during choice deliberation in Anorexia Nervosa. J Neurosci. 2022;42:109–20.
Nakao T, Okada K, Kanba S. Neurobiological model of obsessive-compulsive disorder: evidence from recent neuropsychological and neuroimaging findings. Psychiatry Clin Neurosci. 2014;68:587–605.
Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci. 2013;16:1068–76.
Campagnola L, Seeman SC, Chartrand T, Kim L, Hoggarth A, Gamlin C, et al. Local connectivity and synaptic dynamics in mouse and human neocortex. Science. 2022;375:eabj5861.
Xu H, Jeong HY, Tremblay R, Rudy B. Neocortical somatostatin-expressing GABAergic interneurons disinhibit the thalamorecipient layer 4. Neuron. 2013;77:155–67.
Zhang W, Zhang L, Liang B, Schroeder D, Zhang ZW, Cox GA, et al. Hyperactive somatostatin interneurons contribute to excitotoxicity in neurodegenerative disorders. Nat Neurosci. 2016;19:557–9.
Xu H, Liu L, Tian Y, Wang J, Li J, Zheng J, et al. A disinhibitory microcircuit mediates conditioned social fear in the prefrontal cortex. Neuron. 2019;102:668–682.e665.
Zhang W, Daly KM, Liang B, Zhang L, Li X, Li Y, et al. BDNF rescues prefrontal dysfunction elicited by pyramidal neuron-specific DTNBP1 deletion in vivo. J Mol Cell Biol. 2017;9:117–31.
Rothman JS, Silver RA. NeuroMatic: an integrated open-source software toolkit for acquisition, analysis and simulation of electrophysiological data. Front Neuroinform. 2018;12:14.
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82.
Nentwig TB, Obray JD, Vaughan DT, Chandler LJ. Behavioral and slice electrophysiological assessment of DREADD ligand, deschloroclozapine (DCZ) in rats. Sci Rep. 2022;12:6595.
Nagai Y, Miyakawa N, Takuwa H, Hori Y, Oyama K, Ji B, et al. Deschloroclozapine, a potent and selective chemogenetic actuator enables rapid neuronal and behavioral modulations in mice and monkeys. Nat Neurosci. 2020;23:1157–67.
Cummings KA, Clem RL. Prefrontal somatostatin interneurons encode fear memory. Nat Neurosci. 2020;23:61–74.
Dao NC, Brockway DF, Suresh Nair M, Sicher AR, Crowley NA. Somatostatin neurons control an alcohol binge drinking prelimbic microcircuit in mice. Neuropsychopharmacology. 2021;46:1906–17.
Jiang C, Wang X, Le Q, Liu P, Liu C, Wang Z, et al. Morphine coordinates SST and PV interneurons in the prelimbic cortex to disinhibit pyramidal neurons and enhance reward. Mol Psychiatry. 2021;26:1178–93.
Koppe G, Bruckner G, Brauer K, Hartig W, Bigl V. Developmental patterns of proteoglycan-containing extracellular matrix in perineuronal nets and neuropil of the postnatal rat brain. Cell Tissue Res. 1997;288:33–41.
Bukalo O, Schachner M, Dityatev A. Hippocampal metaplasticity induced by deficiency in the extracellular matrix glycoprotein tenascin-R. J Neurosci. 2007;27:6019–28.
Faissner A, Pyka M, Geissler M, Sobik T, Frischknecht R, Gundelfinger ED, et al. Contributions of astrocytes to synapse formation and maturation—potential functions of the perisynaptic extracellular matrix. Brain Res. Rev. 2010;63:26–38.
Miyata S, Komatsu Y, Yoshimura Y, Taya C, Kitagawa H. Persistent cortical plasticity by upregulation of chondroitin 6-sulfation. Nat. Neurosci. 2012;15:414–22. s411-412
Sigal YM, Bae H, Bogart LJ, Hensch TK, Zhuang X. Structural maturation of cortical perineuronal nets and their perforating synapses revealed by superresolution imaging. Proc Natl Acad Sci USA. 2019;116:7071–6.
Gottschling C, Wegrzyn D, Denecke B, Faissner A. Elimination of the four extracellular matrix molecules tenascin-C, tenascin-R, brevican and neurocan alters the ratio of excitatory and inhibitory synapses. Sci Rep. 2019;9:13939.
Xue YX, Xue LF, Liu JF, He J, Deng JH, Sun SC, et al. Depletion of perineuronal nets in the amygdala to enhance the erasure of drug memories. J Neurosci. 2014;34:6647–58.
Yu Z, Han Y, Hu D, Chen N, Zhang Z, Chen W, et al. Neurocan regulates vulnerability to stress and the anti-depressant effect of ketamine in adolescent rats. Mol Psychiatry. 2022;27:2522–32.
Favuzzi E, Marques-Smith A, Deogracias R, Winterflood CM, Sanchez-Aguilera A, Mantoan L, et al. Activity-dependent gating of parvalbumin interneuron function by the perineuronal net protein brevican. Neuron. 2017;95:639–655 e610.
Fawcett JW, Oohashi T, Pizzorusso T. The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat Rev Neurosci. 2019;20:451–65.
Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, et al. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–94.
Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38.
Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–25.
Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci. 2004;19:1997–2002.
Schoenbaum G, Shaham Y. The role of orbitofrontal cortex in drug addiction: a review of preclinical studies. Biol Psychiatry. 2008;63:256–62.
Chen Y, Wang G, Zhang W, Han Y, Zhang L, Xu H, et al. An orbitofrontal cortex-anterior insular cortex circuit gates compulsive cocaine use. Sci Adv. 2022;8:eabq5745.
Li Y, Simmler LD, Van Zessen R, Flakowski J, Wan JX, Deng F, et al. Synaptic mechanism underlying serotonin modulation of transition to cocaine addiction. Science. 2021;373:1252–6.
Packer AM, Yuste R. Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? J Neurosci. 2011;31:13260–71.
Morawski M, Bruckner MK, Riederer P, Bruckner G, Arendt T. Perineuronal nets potentially protect against oxidative stress. Exp Neurol. 2004;188:309–15.
Banerjee SB, Gutzeit VA, Baman J, Aoued HS, Doshi NK, Liu RC, et al. Perineuronal nets in the adult sensory cortex are necessary for fear learning. Neuron. 2017;95:169–179 e163.
Gogolla N, Caroni P, Luthi A, Herry C. Perineuronal nets protect fear memories from erasure. Science 2009;325:1258–61.
Shi W, Wei X, Wang X, Du S, Liu W, Song J, et al. Perineuronal nets protect long-term memory by limiting activity-dependent inhibition from parvalbumin interneurons. Proc Natl Acad Sci USA. 2019;116:27063–73.
Cichon S, Muhleisen TW, Degenhardt FA, Mattheisen M, Miro X, Strohmaier J, et al. Genome-wide association study identifies genetic variation in neurocan as a susceptibility factor for bipolar disorder. Am J Hum Genet. 2011;88:372–81.
Rybakowski JK, Skibinska M, Leszczynska-Rodziewicz A, Kaczmarek L, Hauser J. Matrix metalloproteinase-9 gene and bipolar mood disorder. Neuromol Med. 2009;11:128–32.
Spijker S, Koskinen MK, Riga D. Incubation of depression: ECM assembly and parvalbumin interneurons after stress. Neurosci Biobehav Rev. 2020;118:65–79.
de Araujo Costa Folha OA, Bahia CP, de Aguiar GPS, Herculano AM, Coelho NLG, de Sousa MBC, et al. Effect of chronic stress during adolescence in prefrontal cortex structure and function. Behav Brain Res. 2017;326:44–51.
Ueno H, Suemitsu S, Murakami S, Kitamura N, Wani K, Matsumoto Y, et al. Juvenile stress induces behavioral change and affects perineuronal net formation in juvenile mice. BMC Neurosci. 2018;19:41.
Yu Z, Chen N, Hu D, Chen W, Yuan Y, Meng S, et al. Decreased density of perineuronal net in prelimbic cortex Is linked to depressive-like behavior in young-aged rats. Front Mol Neurosci. 2020;13:4.
Chen H, He D, Lasek AW. Repeated binge drinking increases perineuronal nets in the insular cortex. Alcohol Clin Exp Res. 2015;39:1930–8.
Lavertu-Jolin M, Chattopadhyaya B, Chehrazi P, Carrier D, Wunnemann F, Leclerc S, et al. Acan downregulation in parvalbumin GABAergic cells reduces spontaneous recovery of fear memories. Mol Psychiatry. 2023;28:2946–63.
Acknowledgements
The research was supported in part by the Ministry of Science and Technology of China (STI2030-Major Projects 2021ZD0202900 and 2019YFA0706201, W.Z.), National Natural Science Foundation of China (32170960, W.Z.).
Author information
Authors and Affiliations
Contributions
W.Z. conceived and designed the experiments. Z.H., X.W., J.T., Y.W, Y.F, J.D., and W.Z. performed the experiments. Z.H., J.T., Y.F. and W.Z. analyzed and interpreted the data. W.Z. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, Z., Wei, X., Tian, J. et al. A disinhibitory microcircuit of the orbitofrontal cortex mediates cocaine preference in mice. Mol Psychiatry (2024). https://doi.org/10.1038/s41380-024-02579-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-024-02579-5