Abstract
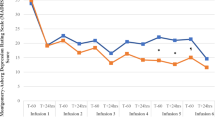
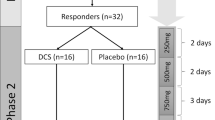
About one-third of patients with depression do not achieve adequate response to current treatment options. Although intravenous and intranasal administrations of (es)ketamine have shown antidepressant properties, their accessibility and scalability are limited. We investigated the efficacy, safety, and tolerability of generic oral esketamine in patients with treatment-resistant depression (TRD) in a randomized placebo-controlled trial with open-label extension. This study consisted of 1) a six-week fixed low-dose treatment phase during which 111 participants received oral esketamine 30 mg or placebo three times a day; 2) a four-week wash-out phase; and 3) an optional six-week open-label individually titrated treatment phase during which participants received 0.5 to 3.0 mg/kg oral esketamine two times a week. The primary outcome measure was change in depressive symptom severity, assessed with the Hamilton Depression Rating Scale (HDRS17), from baseline to 6 weeks. Fixed low-dose oral esketamine when compared to placebo had no benefit on the HDRS17 total score (p = 0.626). Except for dizziness and sleep hallucinations scores, which were higher in the esketamine arm, we found no significant difference in safety and tolerability aspects. During the open-label individually titrated treatment phase, the mean HDRS17 score decreased from 21.0 (SD 5.09) to 15.1 (SD 7.27) (mean difference −6.0, 95% CI −7.71 to −4.29, p < 0.001). Our results suggest that fixed low-dose esketamine is not effective in TRD. In contrast, individually titrated higher doses of oral esketamine might have antidepressant properties.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Access to individual-level data is restricted only to individuals named in the study permit.
References
World Health Organization, Media Centre. Depression Fact Sheet. Updated September 13 2021. https://www.who.int/news-room/fact-sheets/detail/depression. Accessed November 2022.
Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–17.
Bobo WV, Vande Voort JL, Croarkin PE, Leung JG, Tye SJ, Frye MA. Ketamine for treatment-resistant unipolar and bipolar major depression: critical review and implications for clinical practice. Depress Anxiety. 2016;33:698–710.
Kryst J, Kawalec P, Mitoraj AM, Pilc A, Lasón W, Brzostek T. Efficacy of a single and repeated administration of ketamine in unipolar and bipolar depression: a meta-analysis of randomized clinical trials. Pharm Rep. 2020;72:543–62.
Nikolin S, Rodgers A, Schwaab A, Bahji A, Zarate C Jr, Vazquez G, et al. Ketamine for the treatment of major depression: a systematic review and meta-analysis. EClinicalMedicine. 2023;62:102127.
Papakostas GI. Maintaining rapid antidepressant effects following ketamine infusion: a major unmet need. J Clin Psychiatry. 2020;81:19r12859.
Smith-Apeldoorn SY, Veraart JKE, Spijker J, Kamphuis J, Schoevers RA. Maintenance ketamine treatment for depression: a systematic review of efficacy, safety, and tolerability. Lancet Psychiatry. 2022;9:907–21.
Zheng W, Cai D, Xiang Y, Zheng W, Jiang W, Sim K, et al. Adjunctive intranasal esketamine for major depressive disorder: a systematic review of randomized double-blind controlled-placebo studies. J Affect Disord. 2020;265:63–70.
Popova V, Daly EJ, Trivedi M, Cooper K, Lane R, Lim P, et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry. 2019;176:428–38.
Daly EJ, Trivedi MH, Janik A, Li H, Zhang Y, Li X, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76:893–903.
Ross EL, Soeteman DI. Cost-effectiveness of esketamine nasal spray for patients with treatment-resistant depression in the United States. Psychiatr Serv. 2020;71:988–97.
Loo C, Glozier N, Barton D, Baune BT, Mills NT, Fitzgerald P, et al. Efficacy and safety of a 4-week course of repeated subcutaneous ketamine injections for treatment-resistant depression (KADS study): randomised double-blind active-controlled trial. Br J Psychiatry. 2023;223:1–9.
Borner M, Scheithauer W, Twelves C, Maroun J, Wilke H. Answering patients’ needs: oral alternatives to intravenous therapy. Oncologist. 2001;6:12–6.
Schoevers RA, Chaves TV, Balukova SM, Aan het Rot M, Kortekaas R. Oral ketamine for the treatment of pain and treatment-resistant depression. Br J Psychiatry. 2016;208:108–13.
Rosenblat JD, Carvalho AF, Li M, Lee Y, Subramanieapillai M, McIntyre RS. Oral ketamine for depression: a systematic review. J Clin Psychiatry. 2019;80:18r12475.
Smith-Apeldoorn SY, Veraart JKE, Kamphuis J, Van Asselt ADI, Touw DJ, Aan Het Rot M, et al. Oral esketamine for treatment-resistant depression: rationale and design of a randomized controlled trial. BMC Psychiatry. 2019;19:375.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33.
Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–96.
Netherlands National Health Care Institute. August 27 2021. https://www.nvvp.net/nieuws/2021/esketamine-neusspray-in-verzekerd-pakket. Accessed November 2023.
Smith-Apeldoorn SY, Veraart JKE, Ruhé HG, Aan het Rot M, Kamphuis J, De Boer MK, et al. Repeated, low-dose oral esketamine in patients with treatment-resistant depression: pilot study. BJPsych Open. 2021;8:e4.
Andrade C. Oral ketamine for depression, 1: pharmacologic considerations and clinical evidence. J Clin Psychiatry. 2019;80:19f12820.
Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The inventory of depressive symptomatology (IDS). Psychol Med. 1986;26:477–86.
Guy W ECDEU Assessment Manual for Psychopharmacology. Rockville, MD, US Department of Health, Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration, 1976.
Herdman M, Gudex C, Lloyd A, Janssen MF, Kind P, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727–36.
Versteegh MM, Vermeulen KM, Evers SMAA, De Wit GA, Prenger R, Stolk EA. Dutch tariff for the five-level version of EQ-5D. Value Health. 2016;19:343–52.
Levine J, Schooler NR. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacol Bull. 1986;22:343–81.
Aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–45.
Stiglmayr C, Schimke P, Wagner T, Braakmann D, Schweiger U, Sipos V, et al. Development and psychometric characteristics of the Dissociation Tension Scale. J Pers Assess. 2010;92:269–77.
Rossell SL, Schutte MJL, Toh WL, Thomas N, Strauss C, Linszen MMJ, et al. The Questionnaire for psychotic experiences: an examination of the validity and reliability. Schizophr Bull. 2019;45:S78–87.
Koffel E, Watson D. Development and initial validation of the Iowa Sleep Disturbances Inventory. Assessment. 2010;17:423–39.
Twisk J, Bosman L, Hoekstra T, Rijnhart J, Welten M, Heymans M. Different ways to estimate treatment effects in randomized controlled trials. Contemp Clin Trials Commun. 2018;10:80–5.
Lara DR, Bisol LW, Munari LR. Antidepressant, mood stabilizing and precognitive effects of very low dose sublingual ketamine in refractory unipolar and bipolar depression. Int J Neurophsychopharmacol. 2013;16:2111–7.
Glue P, Medlicott NJ, Neehoff S, Surman P, Lam F, Hung N, et al. Safety and efficacy of extended-release ketamine tablets in patients with treatment-resistant depression and anxiety: open label pilot study. Ther Adv Psychopharmacol. 2020;10:2045125320922474.
Arabzadeh S, Hakkikazazi E, Shahmansouri N, Tafakhori A, Ghajar A, Jafarinia M, et al. Does oral administration of ketamine accelerate response to treatment in major depressive disorder? Results of a double-blind controlled trial. J Affect Disord. 2018;235:236–41.
Levinta A, Meshkat S, McIntyre RS, Ho C, Lui LMW, Lee Y, et al. The association between stage of treatment-resistant depression and clinical utility of ketamine/esketamine: a systematic review. J Affect Disord. 2022;318:139–49.
Loo CK, Gálvez V, O’Keefe E, Mitchell PB, Hadzi-Pavlovic D, Leyden J, et al. Placebo-controlled pilot trial testing dose titration and intravenous, intramuscular and subcutaneous routes for ketamine in depression. Acta Psychiatr Scand. 2016;134:48–56.
Jelen LA, Young AH, Stone JM. Ketamine: a tale of two enantiomers. J Psychopharmacol. 2021;35:109–23.
Smith-Apeldoorn SY, Vischjager M, Veraart JKE, Kamphuis J, Aan het Rot M, Schoevers RA. The antidepressant effect and safety of non-intranasal esketamine: a systematic review. J Psychopharmacol. 2022;36:531–44.
Correia-Melo FS, Leal GC, Vieira F, Jesus-Nunes AP, Mello RP, Magnavita G, et al. Efficacy and safety of adjunctive therapy using esketamine or racemic ketamine for adult treatment-resistant depression: a randomized, double-blind, non-inferiority study. J Affect Disord. 2020;264:527–34.
Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affect Disord. 2021;278:542–55.
Leal GC, Bandeira ID, Correia-Melo F, Telles M, Mello RP, Vieira F, et al. Intravenous arketamine for treatment-resistant depression: open-label pilot study. Eur Arch Psychiatry Clin Neurosci. 2021;271:577–82.
Leal GC, Souza-Marques B, Mello RP, Bandeira ID, Caliman-Fontes AT, Carneiro BA, et al. Arketamine as adjunctive therapy for treatment-resistant depression: a placebo-controlled pilot study. J Affect Disord. 2023;330:7–15.
Rush AJ, South C, Jain S, Agha R, Zhang M, Shrestha S, et al. Clinically significant changes in the 17- and 6-Item Hamilton Rating Scales for Depression: A STAR*D Report. Neuropsychiatr Dis Treat. 2021;17:2333–45.
Muthukumaraswamy SD, Forsyth A, Lumley T. Blinding and expectancy confounds in psychedelic randomized controlled trials. Expert Rev Clin Pharm. 2021;14:1133–52.
Dwyer JB, Landeros-Weisenberger A, Johnson JA, Londono Tobon A, Flores JM, Nasir M, et al. Efficacy of intravenous ketamine in adolescent treatment-resistant depression: a randomized midazolam-controlled trial. Am J Psychiatry. 2021;178:352–62.
Zhang MWB, Hong YX, Husain SF, Harris KM, Ho RCM. Analysis of print news media framing of ketamine treatment in the United States and Canada from 2000 to 2015. PloS One. 2017;12:e0173202.
Singh I, Morgan C, Curran V, Nutt D, Schlag A, McShane R. Ketamine treatment for depression: opportunities for clinical innovation and ethical foresight. Lancet Psychiatry. 2017;4:419–26.
Acknowledgements
This study was funded by the Netherlands Organization for Health Research and Development, ZonMw (grant number 80-83600-98-3074, awarded to prof. dr. Schoevers). The authors gratefully acknowledge the contribution of all study participants, research assistants, healthcare professionals, patient and family associations, and all others who contributed to this study. The authors also acknowledge Klaas J. Wardenaar for statistical assistance.
Author contributions
RS obtained funding for the study and was the chief investigator. SS wrote the trial protocol with input from JV, AvA, MahR, and RS. SS, JV, and RS were responsible for general project management. SS, JV, JK, JS, AvdM, and RS were responsible for recruitment and data collection. SS conducted the statistical analysis with input from MahR. All authors contributed to the interpretation of the data. SS drafted the original manuscript, JV, JK, MahR, and RS contributed to the writing of the original manuscript, and all authors contributed to re-drafts. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
JV has received a speakers fee from Janssen Pharmaceuticals, outside the submitted work. RS has received research funding for two randomized clinical trials with generic oral esketamine from the Netherlands Organisation for Health Research & Development and the National Health Care Institute, a speakers fee and an investigator initiated research grant from Janssen Pharmaceuticals, and consultancy fees from Clexio biosciences, GH Research, Beckley PsyTech, and QPS. All other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Smith-Apeldoorn, S.Y., Veraart, J.K.E., Kamphuis, J. et al. Oral esketamine in patients with treatment-resistant depression: a double-blind, randomized, placebo-controlled trial with open-label extension. Mol Psychiatry (2024). https://doi.org/10.1038/s41380-024-02478-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-024-02478-9