Abstract
Kikuchi-Fujimoto disease (KFD) is a reactive lymphadenitis of unclear etiology. To understand the pathogenesis of KFD, we performed targeted RNA sequencing of a well-characterized cohort of 15 KFD specimens with 9 non-KFD lymphadenitis controls. Two thousand and three autoimmunity-related genes were evaluated from archived formalin-fixed paraffin-embedded lymph node tissue and analyzed by a bioinformatics approach. Differential expression analysis of KFD cases compared to controls revealed 44 significantly upregulated genes in KFD. Sixty-eight percent of these genes were associated with the type I interferon (IFN) response pathway. Key component of the pathway including nucleic acid sensors, IFN regulatory factors, IFN-induced antiviral proteins, IFN transcription factors, IFN-stimulated genes, and IFN-induced cytokines were significantly upregulated. Unbiased gene expression pathway analysis revealed enrichment of IFN signaling and antiviral pathways in KFD. Protein–protein interaction analysis and a molecular complex detection algorithm identified a densely interacting 15-gene module of type I IFN pathway genes. Apoptosis regulator IFI6 was identified as a key seed gene. Transcription factor target analysis identified enrichment of IFN-response elements and IFN-response factors. T-cell-associated genes were upregulated while myeloid and B-cell-associated genes were downregulated in KFD. CD123+ plasmacytoid dendritic cells (PDCs) and activated T cells were noted in KFD. In conclusion, KFD is mediated by an aberrant type I interferon response that is likely driven by PDCs and T cells.
Similar content being viewed by others
Introduction
Kikuchi-Fujimoto disease (KFD) is a disorder of unknown etiology that usually presents with cervical lymphadenopathy and systemic symptoms. Extranodal presentations in skin, joints, and solid organs are not uncommon and are associated with recurrence1. Lymphadenopathy and systemic symptoms raise the possibility of infectious, malignant, or connective tissue disorders that can be distinguished by pathologic evaluation of lymph node biopsies, laboratory testing, and appropriate follow-up. Lymph nodes show histiocytic necrosis that is characteristically devoid of granulocytes unlike acute infectious lymphadenitis. Necrotic areas are surrounded by characteristic crescentic histiocytes with cellular debris, T-cell immunoblasts, and plasmacytoid dendritic cells (PDC)2,3. The prominence of activated and sometimes atypical-appearing T cells may mimic a T cell lymphoma, which must be excluded by immunophenotypic and genetic studies. KFD is sometimes histologically indistinguishable from systemic lupus erythematosus (SLE) lymphadenitis which can be excluded by clinical findings and laboratory tests. Sjogren’s Syndrome (SS) is also rarely associated with KFD2.
PDCs are myeloid lineage cells that secrete type I interferon (IFN) in response to a viral infection. PDCs detect pathogen-associated nuclei acids using cytoplasmic and endosomal sensors that trigger the secretion of various IFNs. Type I and II IFN bind to IFN α/β receptors on other cells and induce an antiviral state through the transcription of antiviral IFN-stimulated genes (ISGs) and proteins. IFNs also activate T and B cells to mount an adaptive immune response against pathogens.
Dysregulation of the type I IFN pathway is a common mechanism in many autoimmune disorders4. SLE is the prototypic type I IFN-mediated autoimmune disease5 and shows a strong type I IFN signature3. Although there are many reports evaluating the transcriptome of SLE3,5,6,7, the gene expression profile of KFD has not been studied due to the rarity and limitations of archived material. Here, we evaluated the transcriptome of KFD in archived formalin-fixed paraffin-embedded (FFPE) tissue. We used a bioinformatics approach to show that KFD is strongly associated with a type I IFN response.
Methods
Cases and controls
KFD cases were identified from the pathology archives at the Children’s Hospital of Philadelphia as previously described8. Cases of non-KFD reactive lymphadenitis with necrosis were identified as controls. All samples were lymph node resections. Clinical presentation and follow-up, laboratory studies, hematoxylin and eosin (H&E) stained slides, and immunohistochemical stains were reviewed to confirm the diagnosis. FFPE tissue sections were used for sequencing-based RNA expression analysis. Additional reactive lymph node controls with follicular or interfollicular hyperplasia (n = 11) without prominent necrosis or infectious etiology were also identified9 and used for differential expression analysis (Supplementary Data).
Immunohistochemistry and digital image analysis for CD123 and necrosis
CD123 staining and quantification were performed as described8. Stained slides were digitally scanned at ×20 magnification on an Aperio CS-O slide scanner (Leica Biosystems, Germany). Lymph node tissue and regions of necrosis were manually identified and outlined in Aperio Image Scope to determine the total lymph node area and total area of necrosis, which were then used to calculate the percent of the lymph node that was involved by necrosis (total area of necrosis/total lymph node area).
Gene expression analysis
Targeted RNA-sequencing analysis was performed on archived FFPE tissues using the HTG EdgeSeq platform (HTG Molecular Diagnostics, Arizona)10. This platform was chosen because it showed a high correlation with other RNAseq platforms and a low failure rate with FFPE11,12. The immune-response panel was used for the gene expression analysis of 2003 autoimmunity-related genes and pathways (complete gene list provided in Supplementary File 1) as described9. Briefly, FFPE lymph node tissue from KFD cases and controls were used to prepare DNA libraries and sequenced using high output Next-Seq 500/550 (single end, 75 bp, read length) (Illumina USA). FASTQ files were parsed constructing a gene expression count matrix and analyzed using HTG EdgeSeq Reveal, a web-based data analysis software (version 3.0, default parameters). Differential expression was assessed using DESeq2 package (Python, within Reveal software, 2020 version) and Limma-Voom13.
Pathway enrichment analysis
Pathway enrichment analysis was performed using Metascape, a web-based tool that combines functional enrichment, interactome analysis, gene annotation, and membership search of 40 independent knowledge databases14. It uses the hypergeometric test with Benjamini–Hochberg p value correction and clustering of similar enriched terms in core gene sets: pathway [Reactome Gene Sets, Canonical Pathways, BioCarta Gene Sets, Gene Ontology (GO) Biological Processes, Hallmark Gene Sets, and Kyoto Encyclopedia of Genes and Genomes (KEGG)], functional set (GO Molecular Functions), structural complex [GO Cellular Components, KEGG, and Comprehensive ResoUrce of Mammalian protein complex], and signature modules (immunologic signatures, oncogenic signatures, and chemical and genetic perturbations). Terms with a p value of <0.01, a minimum count of 3 and an enrichment factor of >1.5 (the enrichment factor is the ratio of the observed count to the count expected by chance) were collected and grouped into clusters based on their membership similarities. The whole genome was the default enrichment background in Metascape, but similar results were obtained using only genes of the immune-response panel as the enrichment background.
Protein–protein interaction (PPI) network and module analysis
PPI networks of differentially expressed (DE) genes were analyzed with the threshold (combined score > 0.4) using the STRING tool, which can provide interactions across matched proteins15. Cytoscape v3.8.2 was employed to visualize PPI networks and modules16. Molecular complex detection (MCODE) was applied to extract sub-networks in PPI networks with default algorithms (degree cut-off of 2, node score cut-off of 0.2, K-Core of 2, and max. depth of 100)17. DE genes with a degree centrality of >3.0 were identified as hub genes by using the plug-in CentiScaPe18.
Results
Clinicopathologic characteristics of KFD and controls
Fifteen lymph nodes involved by KFD, and nine controls were selected for transcriptome analysis (Table 1) from a well-characterized pediatric cohort8. The age range and median age of KFD cases and controls were similar. KFD cases showed the expected female predominance with a ratio of 2:1, while controls were male predominant. KFD patients were of predominantly African American ethnicity (53%) compared to controls (33%). East Asian ethnicity was higher in KFD patients (20%) compared to controls (11.1%). The cervical region was the predominant site of involvement in both KFD (73%) and controls (67%). KFD cases showed characteristic histiocytic necrotizing lymphadenitis that was devoid of neutrophils (Fig. 1). Increased histiocytes, CD123+ PDCs, and CD3+ T cells were noted adjacent to necrotic areas. Stains for microorganisms and microbiology culture studies performed on KFD cases were negative. Detailed clinicopathologic characteristics of this cohort were reported previously8. Six KFD patients showed low titers of screening antinuclear autoantibodies but confirmatory tests were negative in four patients. One patient was initially thought to have SLE, but symptoms resolved and antibodies were negative after a few months resulting in a final clinical diagnosis of KFD. Another patient was positive for anti-SSA antibodies but lacked symptoms of SS. Controls showed neutrophilic necrotizing and/or granulomatous (n = 8) or, EBV infectious mononucleosis (n = 1) lymphadenitis patterns that were not consistent with KFD. Microbiology culture of one control was positive for Mycobacterium Avium and Staphylococcus Aureus. One control had a history of Crohn’s disease while another was treated for presumed Kawasaki Disease after biopsy.
Representative KFD case with focus of necrosis surrounded by histiocytes and tingible body macrophages. Low (50×) and high (400×) power images are shown. Inset (1000× oil) shows a crescentic histiocyte. CD163 highlights histiocytes that were also aberrantly positive for myeloperoxidase (MPO). Necrotic areas show apoptotic bodies but no granulocytes as shown by morphology or CD15 immunostaining.
Gene expression signature of KFD
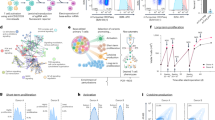
Targeted RNA sequencing of 2003 immune-response and autoimmunity-associated genes was performed using FFPE tissue sections from archived cases and controls. Twenty-three (of the 24) samples passed quality control requirements for RNA degradation and read depth. The one KFD sample that failed RNA quality and read depth metrics was excluded from further analyses. Heat map analyses revealed that KFD cases could be distinguished from controls based on the expression of 94 DE genes (Fig. 2 and Supplementary File 2). Unsupervised clustering analyses revealed that KFD cases were clustered together (Fig. 2B). One reactive lymph node (with necrosis and neutrophils) was clustered along with KFD cases. All laboratory studies for microorganisms were negative and no definite etiology was identified. Many of the genes upregulated in KFD appeared to be IFN-associated and were evaluated in greater detail.
IFN pathway genes are strongly upregulated in KFD
Forty-four genes were significantly upregulated in KFD cases compared to controls (Fig. 3A and Supplementary File 2). Sixty-eight percent of these genes were associated with the IFN pathway (Fig. 3B) noting that IFN-associated genes comprised only 3% of the entire 2003 gene panel (Supplementary File 1). The DE genes spanned every step of the IFN-response pathway (Fig. 5). They could be categorized as: 1. cytosolic nucleic acid sensors (DDX58, IFIH1, ZPB1, and TLR7) that detect foreign viral or self-antigens and initiate the type I IFN pathway; 2. key IFN regulatory factors (IRF7 and IRF9) that mediate transcription of IFNA and B family of genes; 3. IFN-activated transcription factors (STAT1 and STAT2); 4. IFN-induced antiviral proteins (OASL, OAS2, OAS3, and RSAD2); 5. ISGs (ISG20, XAF1, CMPK2, HERC6, SP100, MX1/2, TRIM- and IFIT-family members); 6. Cytokines upregulated by IFN (CXCL10); and 7. miscellaneous genes such as LY6E, which is an IFN-inducible protein that regulates T-cell proliferation19. Although genes from every step of the IFN pathway were strongly upregulated, IFNα and β and their receptors were not among the significantly DE genes in KFD (Supplementary File 2). In contrast to KFD cases, controls did not show strong upregulation of genes of any single pathway (Fig. 3A).
A Volcano plot of significantly differentially expressed (x axis) and upregulated (y axis) genes in KFD (right) and controls (left). Genes in red represent those that satisfied a log-fold change greater than 0.5 and a false discovery rate of 20%. B Box plots of top ten DE genes upregulated in KFD versus controls. Y axis represents log2 transformed transcripts per million. Boxes represent 25–75th percentile of expression and whiskers represent minimum and maximum data points. C H&E (low and higher power) and CD123 stains depicting characteristic clusters of CD123 + PDCs around necrotic areas in a representative case of KFD. D H&E, CD8 and CD30 images show markedly increased CD8+ T cells and rimming of necrotic areas by CD30+ immunoblasts in KFD.
Gene expression analysis also showed upregulation of T-cell-associated genes (CD2, CD3ε, CD3γ, CD3ζ(CD247), CD8, granzymeB, granulysin, and lymphoid activation markers CD38 and CD48) in KFD (Supplementary Fig. 1). B-cell genes CD79a and CD1D and granulocyte marker CD33 were downregulated in KFD. The gene expression profile of immune markers confirmed the morphologic and immunophenotypic findings in KFD (Fig. 3C, D).
PDCs are potent type I IFN producers while activated CD8 T cells are type II IFN producers. Unbiased digital image quantification using CD123 (Fig. 3C) showed increased PDCs in KFD (median 1.64%) compared to controls (median 0.37%). PDCs formed a minor proportion of the overall cellularity in most KFD cases and there were many KFD cases where proportion of PDCs were not different from controls (Supplementary Fig. 3). However, all KFD cases showed marked upregulation of IFN-associated genes (Fig. 3A, B). We considered the possibility that differences in proportion of necrotic areas could account for differences in gene expression. However, proportion of necrotic areas was not significantly different between KFD and controls (Supplementary Fig. 7 left). Necrosis was also not correlated with CD123 levels (Supplementary Fig. 7 right). Similar results were noted even when reactive lymphadenitis controls with follicular or interfollicular hyperplasia9 were used for DE analysis. Although CD123 (IL3RA gene) expression levels were not different between KFD and reactive nodes with hyperplasia (Supplementary Fig. 4), marked upregulation of type I IFN-related genes were noted (Supplementary Figs. 5 and 6).
IFN-signaling pathways are enriched in KFD
We then used an unbiased bioinformatics approach to identify the dominant signaling pathways that are upregulated in KFD. Pathway enrichment analysis of DE genes identified core gene sets of the IFN and immune-response pathways (Fig. 4A). The most significantly enriched pathways were those of type I IFN and antiviral response. Other pathways that were also enriched included the adaptive immune response, TLR7 signaling, viral pathogen response, and type II IFN-signaling pathways. Bacterial and mycobacterial signaling pathways were also highlighted due to the downregulation of associated genes in infectious and granulomatous lymphadenitis controls. A network plot revealed the major pathways by significance and the close connections between them (Fig. 4B).
A Bar graph of enriched terms across input gene lists, colored by p values. B Network of enriched terms colored by cluster ID, where hub genes that share the same cluster ID are typically close to each other. Size of hubs is directly proportional to statistical significance. C Protein–protein interaction enrichment and MCODE analysis identify dense networks of key IFN proteins. IFI6 was identified as a key seed gene.
Protein–protein interaction (PPI) network analysis of DE genes
PPI enrichment analysis of DE genes identified networks of physically interacting partners (Fig. 4B). Two densely interacting networks were identified (Fig. 4C) by the MCODE algorithm. MCODE1 comprised 15 genes with centrality degrees ≥13 (RSAD2, OAS3, OAS2, OASL, IFI6, IFIT3, IFIT1, ISG20, STAT2, XAF1, MX1, IRF7, IFITM1, MX2, and IFI27). IFI6 was identified as a key seed gene for this network. MCODE1 was enriched in Reactome gene sets of type I IFN signaling (R-HSA-909733) and, IFN signaling (R-HSA-913531), and the GO set of defense response to symbiont (GO:014056). Another network MCODE2 with weaker centrality degrees≥3 was identified. MCODE2 comprised 8 genes (DTX3L, UBA7, HERC6, SOCS1, TRIM14, SP100, GBP1 and TRIM22). MCODE2 was enriched in Reactome gene sets of IFN signaling (R-HSA-913531), IFN signaling (R-HAS-877300) and cytokine signaling (R_HSA-1280215). Transcription factor analysis by Metascape identified IFN-stimulated response elements and IFN-response factors (Supplementary Fig. 2).
Discussion
RNAseq of archived FFPE to investigate rare disorders
KFD is a lymphadenitis of hitherto unclear etiology. We previously investigated the immune repertoire and potential viral etiology of KFD using DNA and RNAseq8. Here, we successfully utilized archived FFPE tissue to analyze the transcriptome of KFD. The presence of necrosis and age of the archived sample were not limiting factors for this probe-based targeted gene approach that has a lower quality control failure rate compared to other RNA-sequencing methods10. The lack of a separate RNA isolation step also minimizes extraction or amplification bias. Ninety-nine percent of samples passed manufacturer-recommended quality metrics. Expected upregulation of CD3+ CD8+ T-cell markers and downregulation of myeloid and B-cell markers20,21 served as internal controls. We showed that KFD is associated with a type I IFN-response akin to SLE. Similar methodologies can be used to investigate the etiology of other rare inflammatory and autoimmune disorders using FFPE tissue.
IFN pathway activation in KFD and autoimmune disorders
IFN-response pathways are activated by the presence of nucleic acids in the cytosol or endosome (Fig. 5). Type I and II IFNs secreted by affected cells induce an antiviral state in adjacent cells through the expression of ISGs. We found that many components of the IFN-response pathway were strongly upregulated in KFD. The results cannot be entirely attributed to necrosis, histiocytes or PDCs since our controls showed the same features. Similar results were obtained even when different reactive controls or analysis methods were used, reflecting the strength of the association. Among the significantly upregulated genes were DDX58/RIG-I and IFIH1/MDA5 which detect cytosolic double-stranded RNA, and ZBP1/DAI which detects double-stranded Z DNA and Z RNA. TLR7, a sensor of single-stranded RNA in endosomes, was also upregulated. Cytosolic and endosomal nuclei sensors activate IFN A/B transcription through IRF3 or IRF7. Though IRF7 was upregulated in KFD, type I/II IFN (α,β,γ,ε) or their receptors IFNAR1/2 were not among the upregulated genes. However, several downstream members of the IFN pathway such as STAT1/2 and their transcription factor IRF9 were upregulated. Signaling through this pathway results in expression of many ISGs and antiviral proteins. Our analysis showed that the upregulated genes formed a densely interacting network with apoptosis regulator IFI6 as a seed gene. The network included ISGs ISG20-, XAF1-, MX1/2-, CMPK2-, TRIM-, and IFIT-family members, antiviral protein RSAD2, and the OAS family of enzymes that sense double-stranded viral RNA. These proteins amplify the IFN response through autocrine and paracrine signaling.
Exogenous nucleic acids from microbes and endogenous nucleic acids from apoptotic cells are recognized by endosomal Toll-like receptors (TLR)7 or 9, RNA-binding cytosolic receptors MDA5/RIG-I, and DNA-binding receptors ZBP1/IFI16. These pathways activate Interferon regulatory factors (IRF)3 or IRF7 which induces the expression of type I IFNs. Type I and II IFN are secreted and bind to cells that express the interferon-alpha receptor (IFNAR)1/2 which activates the signal transducer and activator of transcription 1 (STAT1)-STAT2-IRF9 signaling complex. Binding of this complex to IFN-stimulated response elements (ISREs) in gene promoters leads to the induction of a cascade of IFN-stimulated genes (ISGs) and induces an inflammatory antiviral state. Type I IFNs also modulate adaptive immunity through increased antigen presentation by macrophages, antibody production by B cells, cytotoxic function by T cells that result in inflammation and apoptosis. Positive feedback loops amplify the IFN response. Genes upregulated in KFD are underlined here.
Cell types underlying IFN signaling in KFD
PDCs are type I IFN-producing cells that are increased in KFD22 and may underlie upregulation of IFN-response pathway. However, they form just one component of the IFN-response pathway that also involves other cell types such as histiocytes and lymphocytes (Fig. 5). KFD contains increased histiocytes that may play a role in the amplification of the IFN response. Activated CD8+ cytotoxic T cells are type II IFN-producing cells that are increased in KFD20. Our controls of reactive lymph nodes with necrosis (ofinfectious etiology) were selected to control for necrosis, lymphocytes, and PDCs. This allowed us to isolate the signaling pathways that are specific to KFD. The upregulation of several steps of IFN response that occur in different cells indicate that several cell types are involved in upregulation of IFN-related genes in KFD. Hence, histiocytes, lymphocytes, and PDCs may all play a functional role in the pathogenesis of KFD in addition to serving as morphological markers.
Etiology of IFN signaling in KFD and similarities with SLE
While it is clear that there is strong upregulation of IFN pathway in KFD, the initiating signal could not be determined. The source of nucleic acids that trigger cytosolic and endosomal sensors could be exogenous or endogenous. We previously investigated the possible role of infectious agents by RNA sequencing of KFD lymph nodes8 but detect known or novel organisms in KFD. There are strong similarities between genes upregulated in KFD and autoimmune diseases such as SLE6,7. For example, cytosolic sensors DDX58 and IFIH1 are upregulated in circulating monocytes from childhood-onset SLE23. Defective clearance of apoptotic cells in SLE and release of nuclear antigens is also hypothesized to be the basis of autoimmune inflammatory response in SLE24. Hence, it is possible that apoptotic cells may be the cause rather than the consequence of aberrant IFN response in KFD. Analyzing KFD cases in early proliferative or late xanthomatous phase may provide information on sequence of events. However, we did not have sufficient cases with early or late morphology to perform DE analysis.
In contrast to SLE, IFNα and β family members were not upregulated in KFD. High serum IFN levels seen in SLE are hypothesized to be from cutaneous PDCs3,25 or genetic polymorphisms26. The lack of IFN upregulation in KFD could be due to the transient nature of the inciting event in KFD compared to the chronic immune stimulation in SLE. Another intriguing possibility is that KFD could be a ‘forme fruste’ of SLE27 and SS2 with weaker activation of IFN pathway. The presence of antinuclear and anti-SSA antibodies in two KFD cases who did not meet diagnostic clinical criteria for SLE or SS supports this hypothesis. A larger multi-institutional cohort of KFD and SLE cases is needed to address this question.
Data availability
Data generated or analyzed during this study are included in this published article (and its supplementary information files). Any other data will be provided on reasonable request to the corresponding author.
References
Song, J. Y. et al. Clinical outcome and predictive factors of recurrence among patients with Kikuchi’s disease. Int. J. Infect. Dis. 13, 322–326 (2009).
Zhang, J. et al. Kikuchi-Fujimoto disease associated with Sjogren’s syndrome: a case report and review of the literature. Int. J. Clin. Exp. Med. 8, 17061–17066 (2015).
Kunz, M. & Ibrahim, S. M. Cytokines and cytokine profiles in human autoimmune diseases and animal models of autoimmunity. Mediators Inflamm. 2009, 979258 (2009).
Psarras, A., Emery, P. & Vital, E. M. Type I interferon-mediated autoimmune diseases: pathogenesis, diagnosis and targeted therapy. Rheumatology 56, 1662–1675 (2017).
Bengtsson, A. A. et al. Activation of type I interferon system in systemic lupus erythematosus correlates with disease activity but not with antiretroviral antibodies. Lupus 9, 664–671 (2000).
Baechler, E. C. et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl Acad. Sci. USA 100, 2610–2615 (2003).
Han, G. M. et al. Analysis of gene expression profiles in human systemic lupus erythematosus using oligonucleotide microarray. Genes Immun. 4, 177–186 (2003).
Nelson, N. D. et al. Characterization of plasmacytoid dendritic cells, microbial sequences, and identification of a candidate public T-cell clone in Kikuchi-Fujimoto disease. Pediatr. Dev. Pathol. 24, 193–205 (2021).
Wing, A. et al. Transcriptome and unique cytokine microenvironment of Castleman disease. Mod. Pathol. https://doi.org/10.1038/s41379-021-00950-3 (2021).
Qi, Z. et al. Reliable gene expression profiling from small and hematoxylin and eosin-stained clinical formalin-fixed, paraffin-embedded specimens using the HTG EdgeSeq platform. J. Mol. Diagn. 21, 796–807 (2019).
Godoy, P. M. et al. Comparison of reproducibility, accuracy, sensitivity, and specificity of miRNA quantification platforms. Cell Rep. 29, 4212–4222.e4215 (2019).
Zhang, L. et al. Cross-platform comparison of immune-related gene expression to assess intratumor immune responses following cancer immunotherapy. J. Immunol. Methods. 494, 113041 (2021).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019).
Szklarczyk, D. et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 43, D447–D452 (2015).
Cline, M. S. et al. Integration of biological networks and gene expression data using Cytoscape. Nat. Protoc. 2, 2366–2382 (2007).
Bader, G. D. & Hogue, C. W. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinform. 4, 2 (2003).
Scardoni, G., Petterlini, M. & Laudanna, C. Analyzing biological network parameters with CentiScaPe. Bioinformatics 25, 2857–2859 (2009).
Yu, J., Liang, C. & Liu, S. L. Interferon-inducible LY6E protein promotes HIV-1 infection. J. Biol. Chem. 292, 4674–4685 (2017).
Tabata, T. et al. Characteristic distribution pattern of CD30-positive cytotoxic T cells aids diagnosis of Kikuchi-Fujimoto disease. Appl. Immunohistochem. Mol. Morphol. 26, 274–282 (2018).
Chamulak, G. A., Brynes, R. K. & Nathwani, B. N. Kikuchi-Fujimoto disease mimicking malignant lymphoma. Am. J. Surg. Pathol. 14, 514–523 (1990).
Pilichowska, M. E., Pinkus, J. L. & Pinkus, G. S. Histiocytic necrotizing lymphadenitis (Kikuchi-Fujimoto disease): lesional cells exhibit an immature dendritic cell phenotype. Am. J. Clin. Pathol. 131, 174–182 (2009).
Wahadat, M. J. et al. Type I IFN signature in childhood-onset systemic lupus erythematosus: a conspiracy of DNA- and RNA-sensing receptors? Arthritis Res. Ther. 20, 4 (2018).
Mahajan, A., Herrmann, M. & Munoz, L. E. Clearance deficiency and cell death pathways: a model for the pathogenesis of SLE. Front. Immunol. 7, 35 (2016).
Braunstein, I., Klein, R., Okawa, J. & Werth, V. P. The interferon-regulated gene signature is elevated in subacute cutaneous lupus erythematosus and discoid lupus erythematosus and correlates with the cutaneous lupus area and severity index score. Br. J. Dermatol. 166, 971–975 (2012).
Niewold, T. B., Hua, J., Lehman, T. J., Harley, J. B. & Crow, M. K. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes. Immun. 8, 492–502 (2007).
Dorfman, R. F. & Berry, G. J. Kikuchi’s histiocytic necrotizing lymphadenitis: an analysis of 108 cases with emphasis on differential diagnosis. Semin. Diagn. Pathol. 5, 329–345 (1988).
Acknowledgements
We would like to thank Ms. Rachel Olson, Mr. Brian Lockhart, and the Division of Hematopathology for their support. We would like to thank Drs. Eline Luning Prak, Adam Bagg, Bob Doms, and Dale Frank for comments on the manuscript and scientific discussions. Copy editing of the manuscript was performed by the Durnam Consulting Group. The study was funded in part by an autoimmune disease grant from HTG Molecular Diagnostics, Inc.
Author information
Authors and Affiliations
Contributions
V.P. performed study concept and design; V.P., E.Y.L., J.X., D.T.T., E.B., and N.R. performed writing, review, and revision of the paper; V.P., J.X., N.D.N., E.Y.L., D.T.T., and K.T. provided acquisition, analysis and interpretation of data, and statistical analysis; All authors read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was approved by the CHOP Institutional Review Board (IRB 16-013199) and performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Li, E.Y., Xu, J., Nelson, N.D. et al. Kikuchi-Fujimoto disease is mediated by an aberrant type I interferon response. Mod Pathol 35, 462–469 (2022). https://doi.org/10.1038/s41379-021-00992-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00992-7