Abstract
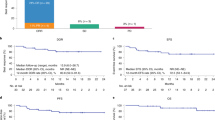
CD19 CAR T-cell (CAR-T) therapy is commonly administered to patients with relapsed or refractory large B-cell lymphomas (LBCL), but salvage or bridging therapy can sometimes lead to a complete response (CR) prior to infusion. Limited studies have assessed the outcomes of patients infused in CR. A total of 134 patients with LBCL in CR prior to CAR-T infusion were identified from the CIBMTR registry, with median prior lines of therapy of 3 (range 2–9). At two years post-infusion, the probability of progression-free survival was 43.5% (95% CI 34.4–52.8) and the probability of overall survival was 63.8% (95% CI 54.4–72.6). The cumulative incidence rates of non-relapse mortality and relapse/progression at two years were 9.2% (95% CI 4.5–15.4) and 47.3% (95% CI 38.2–56.6), respectively. The rate of grade 3 or higher cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) were 2.2% and 8.2%, respectively. In summary, CAR-T in heavily pretreated patients with LBCL who are in CR following two or more lines of prior therapy demonstrate that a subset of patients may remain free of progression at two years. Additionally, the toxicity profile was impressive with very low rates of grade 3 CRS and ICANS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The CIBMTR makes its publication analysis datasets freely available to the public for secondary analysis while safeguarding the privacy of participants and protecting confidential and proprietary data: https://www.cibmtr.org/referencecenter/publist/pubdsdownload.
References
Sehn LH, Salles G. Diffuse large B-cell lymphoma. N Engl J Med. 2021;384:842–58.
Kanate AS, Majhail N, DeFilipp Z, Dhakal B, Dholaria B, Hamilton B, et al. Updated indications for immune effector cell therapy: 2023 guidelines from the American society for transplantation and cellular therapy. Transpl Cell Ther. 2023;29:594–7.
Kanate AS, Majhail NS, Savani BN, Bredeson C, Champlin RE, Crawford S, et al. Indications for hematopoietic cell transplantation and immune effector cell therapy: guidelines from the american society for transplantation and cellular therapy. Biol Blood Marrow Transpl. 2020;26:1247–56.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-Cell therapy in refractory large B-cell lymphoma. N. Engl J Med. 2017;377:2531–44.
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl J Med. 2018;378:439–48.
Abramson, Palomba JS, Gordon LI ML, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–52.
Westin JR, Oluwole OO, Kersten MJ, Miklos DB, Perales MA, Ghobadi A, et al. Survival with axicabtagene ciloleucel in large B-cell lymphoma. N Engl J Med. 2023;389:148–57.
Epperla N, Kumar A, Abutalib SA, Awan FT, Chen YB, Gopal AK, et al. ASTCT clinical practice recommendations for transplantation and cellular therapies in diffuse large B cell lymphoma. Transpl Cell Ther. 2023;29:548–55.
Beaupierre A, Lundberg R, Marrero L, Jain M, Wang T, Alencar MC. Management across settings: an ambulatory and community perspective for patients undergoing CAR T-cell therapy in multiple care settings. Clin J Oncol Nurs. 2019;23:27–34.
Jallouk AP, Gouni S, Westin J, Feng L, Mistry H, Steiner RE, et al. Axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma patients in complete metabolic response. Haematologica. 2023;108:1163–7.
Bishop MR, Maziarz RT, Waller EK, Jager U, Westin JR, McGuirk JP, et al. Tisagenlecleucel in relapsed/refractory diffuse large B-cell lymphoma patients without measurable disease at infusion. Blood Adv. 2019;3:2230–6.
Wudhikarn K, Tomas AA, Flynn JR, Devlin SM, Brower J, Bachanova V, et al. Low toxicity and excellent outcomes in patients with DLBCL without residual lymphoma at the time of CD19 CAR T-cell therapy. Blood Adv. 2023;7:3192–8.
Landsburg DJ, Frigault M, Heim M, Foley SR, Hill BT, Ho CM, et al. Real-world outcomes for patients with relapsed or refractory (R/R) aggressive B-cell non-hodgkin’s lymphoma (aBNHL) treated with commercial tisagenlecleucel: subgroup analyses from the Center for International Blood and Marrow Transplant Research (CIBMTR) registry. Blood. 2022;140:S1584–7.
Locke FL, Rossi JM, Neelapu SS, Jacobson CA, Miklos DB, Ghobadi A, et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020;4:4898–911.
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–68.
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transpl. 2019;25:625–38.
Jacobson CA, Locke FL, Ma L, Asubonteng J, Hu ZH, Siddiqi T, et al. Real-world evidence of axicabtagene ciloleucel for the treatment of large B cell lymphoma in the United States. Transpl Cell Ther. 2022;28:581.e1–e8.
Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US Lymphoma CAR T Consortium. J Clin Oncol. 2020;38:3119–28.
Iacoboni G, Villacampa G, Martinez-Cibrian N, Bailen R, Lopez Corral L, Sanchez JM, et al. Real-world evidence of tisagenlecleucel for the treatment of relapsed or refractory large B-cell lymphoma. Cancer Med. 2021;10:3214–23.
Neelapu SS, Jacobson CA, Ghobadi A, Miklos DB, Lekakis LJ, Oluwole OO, et al. Five-year follow-up of ZUMA-1 supports the curative potential of axicabtagene ciloleucel in refractory large B-cell lymphoma. Blood. 2023;141:2307–15.
Shadman M, Pasquini M, Ahn KW, Chen Y, Turtle CJ, Hematti P, et al. Autologous transplant vs chimeric antigen receptor T-cell therapy for relapsed DLBCL in partial remission. Blood. 2022;139:1330–9.
Hamadani M, Gopal AK, Pasquini M, Kim S, Qiu X, Ahmed S, et al. Allogeneic transplant and CAR-T therapy after autologous transplant failure in DLBCL: a noncomparative cohort analysis. Blood Adv. 2022;6:486–94.
Hamadani M, Hari PN, Zhang Y, Carreras J, Akpek G, Aljurf MD, et al. Early failure of frontline rituximab-containing chemo-immunotherapy in diffuse large B cell lymphoma does not predict futility of autologous hematopoietic cell transplantation. Biol Blood Marrow Transpl. 2014;20:1729–36.
Acknowledgements
The CIBMTR is supported primarily by the Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); 75R60222C00011 from the Health Resources and Services Administration (HRSA); N00014-21-1-2954 and N00014-23-1-2057 from the Office of Naval Research; Support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, Gateway for Cancer Research, Pediatric Transplantation and Cellular Therapy Consortium and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc.; Adaptimmune; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Allogene; Allovir, Inc.; Amgen, Inc.; Angiocrine; Astellas Pharma US; Atara Biotherapeutics; BeiGene; bluebird bio, inc.; Bristol Myers Squibb Co.; CareDx Inc.; CSL Behring; CytoSen Therapeutics, Inc.; Elevance Health; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Gamida-Cell, Ltd.; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc.; Karius; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Mallinckrodt Pharmaceuticals; Merck & Co.; Mesoblast; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; OptumHealth; Orca Biosystems, Inc.; Ossium Health, Inc.; Pfizer, Inc.; Pharmacyclics, LLC, An AbbVie Company; PPD Development, LP; Regimmune; Sanofi; Sarah Cannon; Sobi, Inc.; Stemcyte; Takeda Pharmaceuticals; Talaris Therapeutics; Vertex Pharmaceuticals; Vor Biopharma Inc.; Xenikos BV. TPW is a K12 Scholar supported by the National Cancer Institute of the National Institutes of Health under Award Number K12CA226330. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Conception and design: Trent Wang, Antonio Jimenez Jimenez, Kwang W. Ahn and Mehdi Hamadani. Financial support: CIBMTR. Collection and assembly of data: Manmeet Kaur and Mehdi Hamadani. Data analysis: Mehdi Hamadani, Manmeet Kaur & Kwang W. Ahn. Interpretation: All authors. Manuscript writing: First draft prepared by Trent Wang and Antonio Jimenez. All authors helped revise the manuscript. Final approval of manuscript: All authors.
Corresponding author
Ethics declarations
Competing interests
TPW reports consultancy for: Kite/a Gilead Company, Incyte, Sanofi. He has received research funding from: Syndax/Incyte, AlloVir, Kadmon/Sanofi. MS reports consultancy for: AbbVie, Genentech, AstraZeneca, Pharmacyclics, BeiGene, BMS, MorphoSys/Incyte, Kite, Eli Lilly, Genmab, Mustang Bio, Regeneron, ADC therapeutics, Fate Therapeutics, Janssen and MEI Pharma. He receives research funding: Mustang Bio, BMS, Pharmacyclics, Genentech, AbbVie,TG Therapeutics, BeiGene, AstraZeneca, Genmab, MorphoSys/Incyte, Vincerx. NA reports institutional research funding and consultancy fees from Kite/Gilead; and advisory board funds from Bristol Myers Squibb. JC reports consultancy for: Bristol Myers Squibb and Merit CRO. He serves as a data safety monitoring board member with ICON-Allovir and ICON-Prolacta. He owns stock in Actinium Pharmaceuticals, Bluebird Bio/2Seventy, Dynavax Technologies, aTyr Pharma, Gamida Cell Ltd, Novavax Inc, Ovid Therapeutics, Vaxart, and Veru. AC reports consultancy for: Elsevier, Kite and research funding from: Novartis, Fate Therapeutics, Kite, BMS. MJF reports consulting and advisory roles for Kite/Gilead, BMS, Novartis, JnJ/Legend. He reports research funding from Arcellx, Kite/Gilead, Novartis, Incyte. NG reports Honoraria from: Novartis, Kite, BMS, Caribou, Genentech, Janssen, Seagen, ADC Therapeutics. Brian Hill reports research funding and consultancy from Gilead, Novartis and BMS. TJ receives institutional research funding from” CTI Biopharma, Kartos therapeutics, Incyte, Bristol Myers Squibb; Advisory board participation with: Care Dx, Bristol Myers Squibb, Incyte, Abbvie, CTI, Kite, Cogent Biosciences, Blueprint Medicine, Telios pharma, Protagonist therapeutics; Education activity with Oncology APP, Binaytara Foundation, and ASH Highlights. JEM reports: research funding from Gilead, Atara, CRISPR, Precision Biosciences, and Scripps Research Institute. Dipenkumar Modi reports research funding from: Genentech, ADC Therapeutics, Karyopharm Therapeutics, Genmab. Consultancy: SeaGen, MorphoSys, ADC Therapeutics, Genmab. Honorarium: AstraZeneca, Beigene. MM reports consultancy from: AbbVie, CSL Behring. Peter Riedell reports consultancy and/or advisory board member for AbbVie, Novartis, BMS, ADC Therapeutics, Kite/Gilead, Sana Biotechnology, Nektar Therapeutics, NurixTherapeutics, Intellia Therapeutics, CVS Caremark, Genmab, BeiGene, Janssen, and Pharmacyclics. He has received honoraria from Novartis. Research support from BMS, Kite Pharma, Novartis, MorphoSys, CRISPR Therapeutics, Calibr, Xencor, Fate Therapeutics, AstraZeneca, Genentech, and Tessa Therapeutics. AS report Consultancy: Takeda, BMS/Celgene, Novartis, Janssen, Gilead, Sanofi. Research support: Takeda, BMS/Celgene.Honoraria: Takeda, BMS/Celgene, MSD, Janssen, Amgen, Novartis, Gilead Kite, Sanofi, Roche, Alexion. Speaker’s Bureau: Takeda. AH reports consultancy from: Bristol Myers Squibb, Genentech, Merck, Seattle Genetics, AstraZeneca, ADC Therapeutics, Takeda, Tubulis, Regeneron, Genmab, Pfizer, Caribou Biosciences, Adicet Bio, AddVie, Allogene Therapeutics. He reports research funding from: Bristol Myers Squibb, Genenetech, Merck, Seattle Genetics, KiTE Pharma, Gilead Sciences, AstraZeneca, ADC Therapeutics. CS has served as a paid consultant: Kite/a Gilead Company, Celgene/BMS, Gamida Cell, Karyopharm Therapeutics, Ono Pharmaceuticals, MorphoSys, CSL Behring, Syncopation Life Sciences, CRISPR Therapeutics and GSK. He has received research funds for clinical trials from: Juno Therapeutics, Celgene/BMS, Bristol-Myers Squibb, Precision Biosciences, Actinium Pharmaceuticals, Sanofi-Genzyme and NKARTA. Mehdi Hamadani reports research support/Funding: Takeda Pharmaceutical Company; ADC Therapeutics; Spectrum Pharmaceuticals; Astellas Pharma. Consultancy: ADC Therapeutics, Omeros, CRISPR, BMS, Kite, Abbvie, Caribou, Genmab. Speaker’s Bureau: ADC Therapeutics, AstraZeneca, BeiGene, Kite. DMC: Inc, Genentech, Myeloid Therapeutics, CRISPR. AJJ reports: research funding AbbVie. The other authors reported no conflicts of interest to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, T.P., Ahn, K.W., Shadman, M. et al. Chimeric antigen receptor T-cell infusion for large B-cell lymphoma in complete remission: a center for international blood and marrow transplant research analysis. Leukemia (2024). https://doi.org/10.1038/s41375-024-02242-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41375-024-02242-6