Abstract
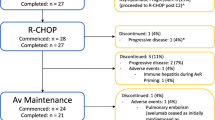
Intensive induction chemotherapy achieves complete remissions (CR) in >60% of patients with acute myeloid leukemia (AML) but overall survival (OS) is poor for relapsing patients not eligible for allogeneic hematopoietic stem cell transplantation (allo-HSCT). Oral azacytidine may be used as maintenance treatment in AML in first remission, but can be associated with substantial side effects, and less toxic strategies should be explored. Twenty AML patients in first CR (CR1) ineligible for allo-HSCT were treated with FDC101, an autologous RNA-loaded mature dendritic cell (mDC) vaccine expressing two leukemia-associated antigens (LAAs). Each dose consisted of 2.5–5 × 106 mDCs per antigen, given weekly until week 4, at week 6, and then monthly, during the 2-year study period. Patients were followed for safety and long-term survival. Treatment was well tolerated, with mild and transient injection site reactions. Eleven of 20 patients (55%) remained in CR, while 4 of 6 relapsing patients achieved CR2 after salvage therapy and underwent allo-HSCT. OS at five years was 75% (95% CI: 50–89), with 70% of patients ≥60 years of age being long-term survivors. Maintenance therapy with this DC vaccine was well tolerated in AML patients in CR1 and was accompanied by encouraging 5-year long-term survival.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data are available upon request from the authors at their discretion.
References
National Cancer Institute. Cancer stat facts: leukemia — acute myeloid leukemia (AML). 2021. https://seer.cancer.gov/statfacts/html/amyl.html.
Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–77.
Dholaria B, Savani BN, Hamilton BK, Oran B, Liu HD, Tallman MS, et al. Hematopoietic cell transplantation in the treatment of newly diagnosed adult acute myeloid leukemia: an evidence-based review from the American Society of Transplantation and Cellular Therapy. Transpl Cell Ther. 2021;27:6–20.
Wei AH, Döhner H, Pocock C, Montesinos P, Afanasyev B, Dombret H, et al. QUAZAR AML-001 trial investigators. oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N Engl J Med. 2020;383:2526–37.
Huls G, Chitu DA, Havelange, Jongen-Lavrencic M, van de Loosdrecht AA, Biemond BJ, et al. Azacitidine maintenance after intensive chemotherapy improves DFS in older AML patients. Blood. 2019;133:1457–64.
Tabah A, Huggar D, Wang ST, Johnson SJ, Copher RM, O´Connell T, et al. Indirect treatment comparison of oral versus injectable azacitidine as maintenance therapy for acute myeloid leukemia. Future Oncol. 2022;18:4089–99
Anguille S, Van de Velde AL, Smits EL, Van Tendeloo VF, Juliusson G, Cools N, et al. Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia. Blood. 2017;130:1713–21.
Van Acker HH, Versteven M, Lichtenegger FS, Roex G, Campillo-Davo D, Lion E, et al. Dendritic cell-based immunotherapy of acute myeloid leukemia. J Clin Med. 2019;8:579.
de Lima M, Roboz GJ, Platzbecker U, Craddock C, Ossenkoppele G. AML and the art of remission maintenance. Blood Rev. 2021;49:100829.
Kumbhari A, Egelston CA, Lee PP, Kim PS. Mature dendritic cells may promote high-avidity tuning of vaccine T cell responses. Front Immunol. 2020;11:584680.
Yu J, Sun H, Cao W, Song Y, Jiang Z. Research progress on dendritic cell vaccines in cancer immunotherapy. Exp Hematol Oncol. 2022;11:3.
Sim EU, Smith A, Szilagi E, Rae F, Ioannou P, Lindsay MH, et al. Wnt-4 regulation by the Wilms’ tumour suppressor gene, WT1. Oncogene. 2002;21:2948–60.
Wang J, Qiu J, Bo L, Wu Z, Zhou A, Xu W, et al. WT1 influences apoptosis and proliferation of immature mice granular cells through regulation of the wnt/β-catenin signal pathway. Cell Mol Biol. (Noisy-le-grand). 2019;65:138–45.
Hosen N, Shirakata T, Nishida S, Yanagihara M, Tsuboi A, Kawakami M, et al. The Wilms’ tumor gene WT1-GFP knock-in mouse reveals the dynamic regulation of WT1 expression in normal and leukemic hematopoiesis. Leukemia. 2007;21:1783–91.
Pozzi S, Geroldi S, Tedone E, Luchetti S, Grasso R, Colombo N, et al. Leukaemia relapse after allogeneic transplants for acute myeloid leukaemia: predictive role of WT1 expression. Br J Haematol. 2013;160:503–9.
Al-Khadairi G, Decock J. Cancer testis antigens and immunotherapy: where do we stand in the targeting of PRAME? Cancers. 2019;11:984.
Epping MT, Wang L, Edel MJ, Carlée L, Hernandez M, Bernards R. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835–47.
Amir AL, van der Steen DM, van Loenen MM, Hagedoorn RS, de Boer R, Kester MD, et al. PRAME-specific Allo-HLA-restricted T cells with potent antitumor reactivity useful for therapeutic T-cell receptor gene transfer. Clin Cancer Res. 2011;17:5615–25.
van den Ancker W, Ruben JM, Westers TM, Wulandari D, Bontkes HJ, Hooijberg E, et al. Priming of PRAME- and WT1-specific CD8+ T cells in healthy donors but not in AML patients in complete remission: implications for immunotherapy. Oncoimmunology. 2013;2:e23971.
Lulla PD, Naik S, Vasileiou S, Tzannou I, Watanabe A, Kuvalekar M, et al. Clinical effects of administering leukemia-specific donor T cells to patients with AML/MDS after allogeneic transplant. Blood. 2021;137:2585–97.
Qin Y, Zhu H, Jiang B, Li J, Lu X, Li L, et al. Expression patterns of WT1 and PRAME in acute myeloid leukemia patients and their usefulness for monitoring minimal residual disease. Leuk Res. 2009;33:384–90.
Zobywalski A, Javorovic M, Frankenberger B, Pohla H, Kremmer E, Bigalke I, et al. Generation of clinical grade dendritic cells with capacity to produce biologically active IL-12p70. J Transl Med. 2007;5:18.
Subklewe M, Geiger C, Lichtenegger FS, Javorovic M, Kvalheim G, Schendel DJ, et al. New generation dendritic cell vaccine for immunotherapy of acute myeloid leukemia. Cancer Immunol Immunother. 2014;63:1093–103.
Herold T, Rothenberg-Thurley M, Grunwald VV, Janke H, Goerlich D, Sauerland MC, et al. Validation and refinement of the revised 2017 European LeukemiaNet genetic risk stratification of acute myeloid leukemia. Leukemia. 2020;34:3161–72.
Lichtenegger FS, Schnorfeil FM, Rothe M, Deiser K, Altmann T, Bücklein VL, et al. Toll-like receptor 7/8-matured RNA-transduced dendritic cells as post-remission therapy in acute myeloid leukaemia: results of a phase I trial. Clin Transl Immunology. 2020;9:e1117.
Khoury HJ, Collins RH Jr, Blum W, Stiff PS, Elias, Lebkowski JS, et al. Immune responses and long-term disease recurrence status after telomerase-based dendritic cell immunotherapy in patients with acute myeloid leukemia. Cancer. 2017;15:3061–72.
Tryggestad AMA, Axcrona K, Axcrona U, Bigalke I, Brennhovd B, Inderberg EM, et al. Long‐term first‐in‐man phase I/II study of an adjuvant dendritic cell vaccine in patients with high‐risk prostate cancer after radical prostatectomy. Prostate. 2022;82:245–53.
Vonk CM, Al Hinai ASA, Hanekamp D, Valk PJM. Molecular minimal residual disease detection in acute myeloid leukemia. Cancers. 2021;13:5431.
Lazzarotto D, Candoni A. The role of Wilms’ tumor gene (WT1) expression as a marker of minimal residual disease in acute myeloid leukemia. J Clin Med. 2022;11:3306.
Author information
Authors and Affiliations
Contributions
YF, GK, DS wrote the protocol. YF, GK, DJ, RWO, HV, IB contributed patient and treatment data for analysis. RG, CG, KP, PUP, FMS, DS collected data and undertook statistical analysis. YF, GK, DS, RG reviewed the data and prepared the manuscript. All authors reviewed the analysis results and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
CG, PP, and DJS are employees of Medigene Immunotherapies GmbH/Medigene AG which hold commercial interests in DC vaccines and served as Sponsor of this study. KP and FMS are previous employees of Medigene Immunotherapies GmbH/Medigene AG and RG is a consultant to Medigene AG.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fløisand, Y., Remberger, M., Bigalke, I. et al. WT1 and PRAME RNA-loaded dendritic cell vaccine as maintenance therapy in de novo AML after intensive induction chemotherapy. Leukemia 37, 1842–1849 (2023). https://doi.org/10.1038/s41375-023-01980-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-023-01980-3