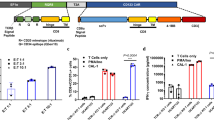
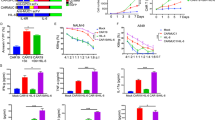
Abstract
Inhibitory myeloid cells and their cytokines play critical roles in limiting chimeric antigen receptor T (CART) cell therapy by contributing to the development of toxicities and resistance following infusion. We have previously shown that neutralization of granulocyte-macrophage colony-stimulating factor (GM-CSF) prevents these toxicities and enhances CART cell functions by inhibiting myeloid cell activation. In this report, we study the direct impact of GM-CSF disruption during the production of CD19-directed CART cells on their effector functions, independent of GM-CSF modulation of myeloid cells. In this study, we show that antigen-specific activation of GM-CSFKO CART19 cells consistently displayed reduced early activation, enhanced proliferation, and improved anti-tumor activity in a xenograft model for relapsed B cell malignancies. Activated CART19 cells significantly upregulate GM-CSF receptors. However, the interaction between GM-CSF and its upregulated receptors on CART cells was not the predominant mechanism of this activation phenotype. GM-CSFKO CART19 cell had reduced BH3 interacting-domain death agonist (Bid), suggesting an interaction between GM-CSF and intrinsic apoptosis pathways. In conclusion, our study demonstrates that CRISPR/Cas9-mediated GM-CSF knockout in CART cells directly ameliorates CART cell early activation and enhances anti-tumor activity in preclinical models.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Sequencing data are available at BioProject PRJNA623000.
References
June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379:64–73.
Anagnostou T, Riaz IB, Hashmi SK, Murad MH, Kenderian SS. Anti-CD19 chimeric antigen receptor T-cell therapy in acute lymphocytic leukaemia: a systematic review and meta-analysis. Lancet Haematol. 2020;7:e816–e826.
Khadka RH, Sakemura R, Kenderian SS, Johnson AJ. Management of cytokine release syndrome: an update on emerging antigen-specific T cell engaging immunotherapies. Immunotherapy. 2019;11:851–7.
Teachey DT, Lacey SF, Shaw PA, Melenhorst JJ, Maude SL, Frey N, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6:664–79.
Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20:31–42.
Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak O, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–54.
Sakemura R, Cox MJ, Hefazi M, Siegler EL, Kenderian SS. Resistance to CART cell therapy: lessons learned from the treatment of hematological malignancies. Leuk Lymphoma. 2021;62:1–18.
Cox MJ, Kenderian SS. Omics analyses provide insights to CART cell therapy resistance. J Transl Genet Genom 2021;5:80–8.
Sterner RM, Kenderian SS. Myeloid cell and cytokine interactions with chimeric antigen receptor-T-cell therapy: implication for future therapies. Curr Opin Hematol. 2020;27:41–48.
Stroncek DF, Ren J, Lee DW, Tran M, Frodigh SE, Sabatino M, et al. Myeloid cells in peripheral blood mononuclear cell concentrates inhibit the expansion of chimeric antigen receptor T cells. Cytotherapy. 2016;18:893–901.
Ruella M, Klichinsky M, Kenderian SS, Shestova O, Ziober A, Kraft DO, et al. Overcoming the immunosuppressive tumor microenvironment of Hodgkin Lymphoma using chimeric antigen receptor T cells. Cancer Disco. 2017;7:1154–67.
Jain MD, Zhao H, Wang X, Atkins R, Menges M, Reid K, et al. Tumor interferon signaling and suppressive myeloid cells associate with CAR T cell failure in large B cell lymphoma. Blood. 2021;137:2621–33.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44.
Shi Y, Liu CH, Roberts AI, Das J, Xu G, Ren G, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don’t know. Cell Res. 2006;16:126–33.
Tugues S, Amorim A, Spath S, Martin-Blondel G, Schreiner B, De Feo D, et al. Graft-versus-host disease, but not graft-versus-leukemia immunity, is mediated by GM-CSF-licensed myeloid cells. Sci Transl Med. 2018;10.
Sterner RM, Sakemura R, Cox MJ, Yang N, Khadka RH, Forsman CL, et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood. 2019;133:697–709.
Sterner RM, Cox MJ, Sakemura R, Kenderian SS. Using CRISPR/Cas9 to knock out GM-CSF in CAR-T cells. J. Vis. Exp. 2019;149:e59629.
Manriquez-Roman C, Siegler EL, Kenderian SS. CRISPR takes the front seat in CART-cell development. BioDrugs. 2021;35:113–24.
Sachdeva M, Duchateau P, Depil S, Poirot L, Valton J. Granulocyte-macrophage colony-stimulating factor inactivation in CAR T-cells prevents monocyte-dependent release of key cytokine release syndrome mediators. J Biol Chem. 2019;294:5430–7.
Zhang J, Roberts AI, Liu C, Ren G, Xu G, Zhang L, et al. A novel subset of helper T cells promotes immune responses by secreting GM-CSF. Cell Death Differ. 2013;20:1731–41.
Li H, Durbin R. Fast and accurate long-read alignment with Burrows–Wheeler transform. Bioinformatics. 2010;26:589–95.
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–303.
Genomes Project C, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. 2015;526:68–74.
Stemmer M, Thumberger T, Del Sol Keyer M, Wittbrodt J, Mateo JL. CCTop: an intuitive, flexible and reliable CRISPR/Cas9 target prediction tool. PLoS One. 2015;10:e0124633.
Brinkman EK, Chen T, Amendola M, van Steensel B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 2014;42:e168–e168.
Iyer V, Boroviak K, Thomas M, Doe B, Riva L, Ryder E, et al. No unexpected CRISPR-Cas9 off-target activity revealed by trio sequencing of gene-edited mice. PLoS Genet. 2018;14:e1007503.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550.
Zhang Y, Li H, Wang X, Gao X, Liu X. Regulation of T cell development and activation by creatine kinase B. PLoS One. 2009;4:e5000.
Juric V, Chen CC, Lau LF. Fas-mediated apoptosis is regulated by the extracellular matrix protein CCN1 (CYR61) in vitro and in vivo. Mol Cell Biol. 2009;29:3266–79.
Yamamoto TN, Lee PH, Vodnala SK, Gurusamy D, Kishton RJ, Yu Z, et al. T cells genetically engineered to overcome death signaling enhance adoptive cancer immunotherapy. J Clin Investig. 2019;129:1551–65.
Michelozzi IM, Gomez-Castaneda E, Pohle RVC, Rodriguez F, Sufi J, Puigdevall P, et al. The Enhanced Functionality of Low-Affinity CD19 CAR T Cells Is Associated with Activation Priming and Polyfunctional Cytokine Phenotype. Blood 2020;136:52–53.
Tschumi BO, Dumauthioz N, Marti B, Zhang L, Schneider P, Mach JP, et al. CART cells are prone to Fas- and DR5-mediated cell death. J Immunother Cancer. 2018;6:71.
Benmebarek MR, Karches CH, Cadilha BL, Lesch S, Endres S, Kobold S. Killing mechanisms of chimeric antigen receptor (CAR) T cells. Int J Mol Sci. 2019;20:1283.
Gomes-Silva D, Mukherjee M, Srinivasan M, Krenciute G, Dakhova O, Zheng Y, et al. Tonic 4-1BB costimulation in chimeric antigen receptors impedes T cell survival and is vector-dependent. Cell Rep. 2017;21:17–26.
Kunkele A, Johnson AJ, Rolczynski LS, Chang CA, Hoglund V, Kelly-Spratt KS, et al. Functional tuning of CARs reveals signaling threshold above which CD8+ CTL antitumor potency is attenuated due to cell Fas-FasL-dependent AICD. Cancer Immunol Res. 2015;3:368–79.
Siegler EL, Simone BW, Sakemura R, Tapper EE, Horvei P, Cox MJ, et al. Efficient gene editing of CART cells with CRISPR-Cas12a for enhanced antitumor efficacy. Blood. 2020;136:6–7.
Ghezraoui H, Piganeau M, Renouf B, Renaud JB, Sallmyr A, Ruis B, et al. Chromosomal translocations are mediated by canonical NHEJ in human cells. Cancer Discov. 2014;4:OF12.
Stadtmauer EA, Cohen AD, Weber K, Lacey SF, Gonzalez VE, Melenhorst JJ, et al. First-in-human assessment of feasibility and safety of multiplexed genetic engineering of autologous T cells expressing NY-ESO -1 TCR and CRISPR/Cas9 gene edited to eliminate endogenous TCR and PD-1 (NYCE T cells) in advanced multiple myeloma (MM) and sarcoma. Blood. 2019;134:49.
Yi Y, Chai X, Zheng L, Zhang Y, Shen J, Hu B, et al. CRISPR-edited CART with GM-CSF knockout and auto secretion of IL6 and IL1 blockers in patients with hematologic malignancy. Cell Disco. 2021;7:27.
Acknowledgements
Mayo Clinic Cancer Center (SK).
Funding
Exact Foundation (SSK), Humanigen (SSK), Mayo Clinic Center for Clinical and Translational Science grant UL1TR002377 (SSK), Mayo Clinic Center for Individualized Medicine (SSK), National Comprehensive Cancer Network (SSK), National Institutes of Health grants K12CA090628 (SSK) and R37CA266344-01 (SSK), Department of Defense grant CA201127 (SSK), and Predolin Foundation (RS and SSK).
Author information
Authors and Affiliations
Contributions
SSK formulated the initial idea; MJC, CMR, RS, and SSK designed experiments; MJC, CMR, RS, EET, ELS, and SS performed experiments; MJC, CMR, RS, and SS analyzed data; MJC, RM, and NS analyzed sequencing data; SAP and NEK contributed reagents; SSK supervised the study. All authors edited and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
SSK is an inventor on patents in the field of CAR immunotherapy that are licensed to Novartis (through an agreement between Mayo Clinic, University of Pennsylvania, and Novartis). MJC, RS, RMS and SSK are inventors on patents in the field of CAR immunotherapy that are licensed to Humanigen (through Mayo Clinic). MH and SSK are inventors on patents in the field of CAR immunotherapy that are licensed to Mettaforge (through Mayo Clinic). SSK receives research funding from Kite, Gilead, Juno, BMS, Novartis, Humanigen, MorphoSys, Tolero, Sunesis/Viracta, Leahlabs, and Lentigen. NEK receives research funding from Acerta Pharma, BMS, Pharmacyclics, MEI Pharma, and Sunesis. NEK has participated in Advisory Board meetings of Cytomx Therapy, Janssen, Juno Therapeutics, Astra Zeneca, and Oncotracker; and on DSMC for Agios and Cytomx Therapeutics. SAP receives research funding from Pharmacyclics, MorphoSys, Janssen, AstraZeneca, TG Therapeutics, BMS, AbbVie, and Ascentage Pharma. SAP has participated in Advisory Board meetings of Pharmacyclics, AstraZeneca, Genentech, Gilead, GlaxoSmithKline, Verastem Oncology, and AbbVie (he was not personally compensated for his participation). CD, DC and OA are employees of Humanigen.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Cox, M.J., Manriquez Roman, C., Tapper, E.E. et al. GM-CSF disruption in CART cells modulates T cell activation and enhances CART cell anti-tumor activity. Leukemia 36, 1635–1645 (2022). https://doi.org/10.1038/s41375-022-01572-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-022-01572-7
This article is cited by
-
Mesenchymal stromal cells with chimaeric antigen receptors for enhanced immunosuppression
Nature Biomedical Engineering (2024)
-
Molecular and therapeutic effect of CRISPR in treating cancer
Medical Oncology (2023)
-
RNA silencing of GM-CSF in CAR-T cells reduces the secretion of multiple inflammatory cytokines
Investigational New Drugs (2023)
-
Safety and efficacy of a novel anti-CD19 chimeric antigen receptor T cell product targeting a membrane-proximal domain of CD19 with fast on- and off-rates against non-Hodgkin lymphoma: a first-in-human study
Molecular Cancer (2023)
-
speedingCARs: accelerating the engineering of CAR T cells by signaling domain shuffling and single-cell sequencing
Nature Communications (2022)