Abstract
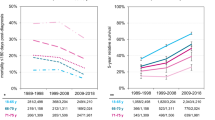
Advances in the understanding of disease biology, drug development, and supportive care have led to improved outcomes in multiple myeloma. Given that these improvements have been reported in clinical trial and referral center populations, questions remain about the generalizability of this observation to patients treated in the community. Contrasting the overall survival experience of 3783 patients seen at Mayo Clinic and 57,654 patients followed in the Surveillance, Epidemiology, and End Results Program (SEER) between 2004 and 2018, we observed different mortality trends across patient populations and subgroups. Early mortality decreased and estimated 5-year overall survival increased over time in both patient populations. Excess mortality (compared to the general population) declined over time in Mayo Clinic patients and remained largely unchanged in SEER patients. Improvements over time were primarily observed in patients with favorable disease characteristics and older patients with multiple myeloma remain a vulnerable population with significant excess mortality compared to the United States general population. Patients with unfavorable disease characteristics have derived disproportionately less benefit from recent advances in the field. Future efforts need to focus on the development of safe and effective therapies for these patients and on increasing timely access to specialized care for patients in the community.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kazandjian D, Landgren O. A look backward and forward in the regulatory and treatment history of multiple myeloma: Approval of novel-novel agents, new drug development, and longer patient survival. Semin Oncol. 2016;43:682–9.
Durie BGM, Hoering A, Abidi MH, Rajkumar SV, Epstein J, Kahanic SP, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389:519–27.
Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28:1122–8.
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–20.
Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319–31.
Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373:621–31.
San-Miguel JF, Hungria VT, Yoon SS, Beksac M, Dimopoulos MA, Elghandour A, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15:1195–206.
Voorhees PM, Kaufman JL, Laubach J, Sborov DW, Reeves B, Rodriguez C, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020;136:936–45.
Heneghan C, Goldacre B, Mahtani KR. Why clinical trial outcomes fail to translate into benefits for patients. Trials. 2017;18:122.
Scher KS, Hurria A. Under-representation of older adults in cancer registration trials: known problem, little progress. J Clin Oncol. 2012;30:2036–8.
Unger JM, Cook E, Tai E, Bleyer A. The role of clinical trial participation in cancer research: barriers, evidence, and strategies. Am Soc Clin Oncol Educ Book. 2016;35:185–98.
U.S. Population Data 1969-2019 with Other Software: (downloaded from SEER Web site); Surveillance, Epidemiology, and End Results (SEER) Program Populations (1969-2019) (www.seer.cancer.gov/popdata), National Cancer Institute, DCCPS, Surveillance Research Program, released February 2021.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Cox DR. Regression models and life-tables. J R Stat Soc Ser B (Methodol). 1972;34:187–220.
Newson RB. Sensible parameters for univariate and multivariate splines. Stata J. 2012;12:479–504. 2012/09/01
Coviello E. STEXPECT: Stata module to compute expected survival. Statistical Software Components 2005. S438201, Boston College Department of Economics, revised 04/10/2005. https://ideas.repec.org/c/boc/bocode/s438201.html.
Dickman PW, Coviello E. Estimating and modeling relative survival. Stata J. 2015;15:186–215. 2015/04/01
Royston P, Sauerbrei W. A new measure of prognostic separation in survival data. Stat Med. 2004;23:723–48.
StataCorp. Stata statistical software: release 16. College Station, TX: StataCorp LLC. 2019.
Boyle EM, Williams L, Blaney P, Ashby C, Bauer M, Walker BA, et al. Improving prognostic assignment in older adults with multiple myeloma using acquired genetic features, clonal hemopoiesis and telomere length. Leukemia. 2021. https://doi.org/10.1038/s41375-021-01320-3. Epub ahead of print. PMID: 34148053.
Pawlyn C, Cairns D, Kaiser M, Striha A, Jones J, Shah V, et al. The relative importance of factors predicting outcome for myeloma patients at different ages: results from 3894 patients in the Myeloma XI trial. Leukemia. 2020;34:604–12.
Cavo M, Pantani L, Petrucci MT, Patriarca F, Zamagni E, Donnarumma D, et al. Bortezomib-thalidomide-dexamethasone is superior to thalidomide-dexamethasone as consolidation therapy after autologous hematopoietic stem cell transplantation in patients with newly diagnosed multiple myeloma. Blood. 2012;120:9–19.
Sonneveld P, Avet-Loiseau H, Lonial S, Usmani S, Siegel D, Anderson KC, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127:2955–62.
Gay F, Larocca A, Wijermans P, Cavallo F, Rossi D, Schaafsma R, et al. Complete response correlates with long-term progression-free and overall survival in elderly myeloma treated with novel agents: analysis of 1175 patients. Blood. 2011;117:3025–31.
Pulte D, Gondos A, Brenner H. Improvement in survival of older adults with multiple myeloma: results of an updated period analysis of SEER data. Oncologist. 2011;16:1600–3.
Thorsteinsdottir S, Dickman PW, Landgren O, Blimark C, Hultcrantz M, Turesson I, et al. Dramatically improved survival in multiple myeloma patients in the recent decade: results from a Swedish population-based study. Haematologica. 2018;103:e412–e415.
Turesson I, Velez R, Kristinsson SY, Landgren O. Patterns of improved survival in patients with multiple myeloma in the twenty-first century: a population-based study. J Clin Oncol. 2010;28:830–4.
Hari P, Romanus D, Luptakova K, Blazer M, Yong C, Raju A, et al. The impact of age and comorbidities on practice patterns and outcomes in patients with relapsed/refractory multiple myeloma in the era of novel therapies. J Geriatr Oncol. 2018;9:138–44.
Larocca A, Bonello F, Gaidano G, D’Agostino M, Offidani M, Cascavilla N, et al. Dose/schedule-adjusted Rd-R vs continuous Rd for elderly, intermediate-fit patients with newly diagnosed multiple myeloma. Blood. 2021;137:3027–36.
Palumbo A, Bringhen S, Mateos MV, Larocca A, Facon T, Kumar SK, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. 2015;125:2068–74.
Acknowledgements
The MMRF kindly provided the data from the CoMMpass study for the purpose of scientific research. These data were generated as part of the Multiple Myeloma Research Foundation Personalized Medicine Initiatives (https://research.themmrf.org and www.themmrf.org).
Author information
Authors and Affiliations
Contributions
MB, BN, and SKK were responsible for the study conception and design; MB, BN, and SKK were involved in the collection and assembly of data; MB and BN performed the statistical analysis; MB, BN, and SKK wrote the manuscript; all authors participated in data analysis and interpretation, critical revision of the manuscript, and provided their final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Binder, M., Nandakumar, B., Rajkumar, S.V. et al. Mortality trends in multiple myeloma after the introduction of novel therapies in the United States. Leukemia 36, 801–808 (2022). https://doi.org/10.1038/s41375-021-01453-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-021-01453-5
This article is cited by
-
Bone marrow stromal cells induce chromatin remodeling in multiple myeloma cells leading to transcriptional changes
Nature Communications (2024)
-
Thromboprophylaxis in multiple myeloma: a case-based review with practical guidelines
Annals of Hematology (2024)
-
Mass Cytometry reveals unique phenotypic patterns associated with subclonal diversity and outcomes in multiple myeloma
Blood Cancer Journal (2023)
-
Use of the European Organisation for Research and Treatment of Cancer multiple myeloma module (EORTC QLQ-MY20): a review of the literature 25 years after development
Blood Cancer Journal (2023)
-
Global disparities in patients with multiple myeloma: a rapid evidence assessment
Blood Cancer Journal (2023)