Abstract
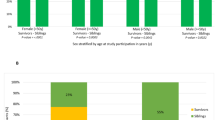
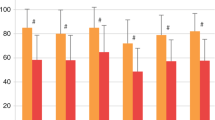
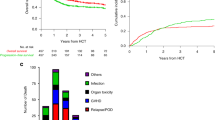
Cumulative burden of chronic health conditions and neurocognitive and physical function were examined among survivors of childhood acute myeloid leukemia (AML) treated with hematopoietic cell transplant (HCT; n = 66) or conventional therapy (CT; n = 67). Survivors and controls underwent a comprehensive clinical assessment, and health conditions were graded using a modified version of the Common Terminology Criteria for Adverse Events. By age 40 years, HCT and CT survivors had an average 17.4 (95% confidence interval [CI] 14.6–20.1) and 9.3 (7.7–11.1) grade 1–4 conditions versus 3.8 (3.3–4.2) in community controls. Compared to controls, HCT survivors had a higher prevalence of hypertriglyceridemia (45.5% vs. 18.3%), hypercholesterolemia (47.0% vs. 30.9%), hypothyroidism (27.3% vs. 4.0%), and primary hypogonadism (p < 0.001). CT survivors had a higher prevalence of cardiomyopathy (11.9% vs. 2.7%) and hypertension (53.7% vs. 44.3%). Neurocognitive impairment was elevated across all domains compared to controls but did not differ by treatment modality. Compared to controls, a higher proportion of HCT survivors had impairments in strength and endurance; whereas flexibility and mobility impairments were noted among CT survivors. Despite successful advances in childhood AML therapy, many therapeutic exposures remain unchanged. These findings support ongoing investigations of novel therapies and strategies to ameliorate the risk of late morbidities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Statistical code is available upon request. Study protocol is available at Clinical Trials.gov (NCT00760656).
References
Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64:83–103.
Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, et al (eds). SEER Cancer Statistics Review, 1975-2017, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2017/, based on November 2019 SEER data submission, posted to the SEER web site, April 2020.
Creutzig U, van den Heuvel-Eibrink MM, Gibson B, Dworzak MN, Adachi S, de Bont E, et al. Diagnosis and management of acute myeloid leukemia in children and adolescents: recommendations from an international expert panel. Blood. 2012;120:3187–205.
Armstrong GT, Chen Y, Yasui Y, Leisenring W, Gibson TM, Mertens AC, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N. Engl J Med. 2016;374:833–42.
Gibson TM, Mostoufi-Moab S, Stratton KL, Leisenring WM, Barnea D, Chow EJ, et al. Temporal patterns in the risk of chronic health conditions in survivors of childhood cancer diagnosed 1970–99: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2018;19:1590–601.
Bhakta N, Liu Q, Ness KK, Baassiri M, Eissa H, Yeo F, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet. 2017;390:2569–82.
Barlogis V, Auquier P, Bertrand Y, Chastagner P, Plantaz D, Poiree M, et al. Late cardiomyopathy in childhood acute myeloid leukemia survivors: a study from the L.E.A. program. Haematologica. 2015;100:e186–9.
Jarfelt M, Andersen NH, Glosli H, Jahnukainen K, Jonmundsson GK, Malmros J, et al. Cardiac function in survivors of childhood acute myeloid leukemia treated with chemotherapy only: a NOPHO-AML study. Eur J Haematol. 2016;97:55–62.
Leung W, Hudson MM, Strickland DK, Phipps S, Srivastava DK, Ribeiro RC, et al. Late effects of treatment in survivors of childhood acute myeloid leukemia. J Clin Oncol. 2000;18:3273–9.
Molgaard-Hansen L, Glosli H, Jahnukainen K, Jarfelt M, Jonmundsson GK, Malmros-Svennilson J, et al. Quality of health in survivors of childhood acute myeloid leukemia treated with chemotherapy only: a NOPHO-AML study. Pediatr Blood Cancer. 2011;57:1222–9.
Molgaard-Hansen L, Skou AS, Juul A, Glosli H, Jahnukainen K, Jarfelt M, et al. Pubertal development and fertility in survivors of childhood acute myeloid leukemia treated with chemotherapy only: a NOPHO-AML study. Pediatr Blood Cancer. 2013;60:1988–95.
Mulrooney DA, Dover DC, Li S, Yasui Y, Ness KK, Mertens AC, et al. Twenty years of follow-up among survivors of childhood and young adult acute myeloid leukemia: a report from the Childhood Cancer Survivor Study. Cancer. 2008;112:2071–9.
Schultz KA, Chen L, Chen Z, Kawashima T, Oeffinger KC, Woods WG, et al. Health conditions and quality of life in survivors of childhood acute myeloid leukemia comparing post remission chemotherapy to BMT: a report from the children’s oncology group. Pediatr Blood Cancer. 2014;61:729–36.
Skou AS, Glosli H, Jahnukainen K, Jarfelt M, Jonmundsson GK, Malmros-Svennilson J, et al. Renal, gastrointestinal, and hepatic late effects in survivors of childhood acute myeloid leukemia treated with chemotherapy only–a NOPHO-AML study. Pediatr Blood Cancer. 2014;61:1638–43.
Wilhelmsson M, Glosli H, Ifversen M, Abrahamsson J, Winiarski J, Jahnukainen K, et al. Long-term health outcomes in survivors of childhood AML treated with allogeneic HSCT: a NOPHO-AML Study. Bone Marrow Transplant. 2019;54:726–36.
Liesner RJ, Leiper AD, Hann IM, Chessells JM. Late effects of intensive treatment for acute myeloid leukemia and myelodysplasia in childhood. J Clin Oncol. 1994;12:916–24.
Leahey AM, Teunissen H, Friedman DL, Moshang T, Lange BJ, Meadows AT. Late effects of chemotherapy compared to bone marrow transplantation in the treatment of pediatric acute myeloid leukemia and myelodysplasia. Med Pediatr Oncol. 1999;32:163–9.
Hudson MM, Ness KK, Nolan VG, Armstrong GT, Green DM, Morris EB, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2011;56:825–36.
Howell C, Bjornard K, Ness K, Alberts N, Armstrong G, Bhakta N, et al. Cohort profile: the St. Jude Lifetime Cohort Study (SJLIFE) for pediatric cancer survivors Int J Epidemiol. 2020. In press.
Feijen EA, Leisenring WM, Stratton KL, Ness KK, van der Pal HJ, Caron HN, et al. Equivalence ratio for daunorubicin to doxorubicin in relation to late heart failure in survivors of childhood cancer. J Clin Oncol. 2015;33:3774–80.
Green DM, Nolan VG, Goodman PJ, Whitton JA, Srivastava D, Leisenring WM, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2014;61:53–67.
Howell RM, Smith SA, Weathers RE, Kry SF, Stovall M. Adaptations to a generalized radiation dose reconstruction methodology for use in epidemiologic studies: an update from the MD Anderson late effect group. Radiat Res. 2019;192:169–88.
Hudson MM, Ehrhardt MJ, Bhakta N, Baassiri M, Eissa H, Chemaitilly W, et al. Approach for classification and severity grading of long-term and late-onset health events among childhood cancer survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomark Prev. 2017;26:666–74.
Strauss E, Sherman EM, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Third ed. New York, NY: Oxford University Press; 2006.
Conners CK. Conners’ Continuous Performance Test II. North Tonawanda, NY: Multi-Health Systems; 2001.
Reynolds C, Voress JK. Test of memory and learning. Second ed. Austin, TX: PRO-ED; 2007.
Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. Second ed. San Antonio, TX: The Psychological Corporation; 2000.
Wechsler D. Wechsler Adult Intelligence Scale. Third ed. San Antonio, TX: Psychological Corporation; 1997.
Lipkin DP, Scriven AJ, Crake T, Poole-Wilson PA. Six minute walking test for assessing exercise capacity in chronic heart failure. Br Med J. 1986;292:653–5.
Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–8.
Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S. Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil. 1985;66:69–74.
Muscular weakness assessment: use of normal isometric strength data. The National Isometric Muscle Strength (NIMS) Database Consortium. Arch Phys Med Rehabil. 1996;77:1251–5.
Moseley AM, Crosbie J, Adams R. Normative data for passive ankle plantarflexion–dorsiflexion flexibility. Clin Biomech. 2001;16:514–21.
Shephard RJ, Berridge M, Montelpare W. On the generality of the “sit and reach” test: an analysis of flexibility data for an aging population. Res Q Exerc Sport. 1990;61:326–30.
Nashner LM, Peters JF. Dynamic posturography in the diagnosis and management of dizziness and balance disorders. Neurol Clin. 1990;8:331–49.
Dong H, Robison LL, Leisenring WM, Martin LJ, Armstrong GT, Yasui Y. Estimating the burden of recurrent events in the presence of competing risks: the method of mean cumulative count. Am J Epidemiol. 2015;181:532–40.
Geskus RB. Cause-specific cumulative incidence estimation and the fine and gray model under both left truncation and right censoring. Biometrics. 2011;67:39–49.
Rubnitz JE, Lacayo NJ, Inaba H, Heym K, Ribeiro RC, Taub J, et al. Clofarabine can replace anthracyclines and etoposide in remission induction therapy for childhood acute myeloid leukemia: the AML08 multicenter, randomized phase iii trial. J Clin Oncol. 2019;37:2072–81.
Rubnitz JE, Inaba H, Dahl G, Ribeiro RC, Bowman WP, Taub J, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11:543–52.
Dluzniewska A, Balwierz W, Armata J, Balcerska A, Chybicka A, Kowalczyk J, et al. Twenty years of Polish experience with three consecutive protocols for treatment of childhood acute myelogenous leukemia. Leukemia. 2005;19:2117–24.
Alexander TB, Wang L, Inaba H, Triplett BM, Pounds S, Ribeiro RC, et al. Decreased relapsed rate and treatment-related mortality contribute to improved outcomes for pediatric acute myeloid leukemia in successive clinical trials. Cancer.2017;123:3791–8.
Creutzig U, Zimmermann M, Lehrnbecher T, Graf N, Hermann J, Niemeyer CM, et al. Less toxicity by optimizing chemotherapy, but not by addition of granulocyte colony-stimulating factor in children and adolescents with acute myeloid leukemia: results of AML-BFM 98. J Clin Oncol. 2006;24:4499–506.
Mulrooney DA, Hyun G, Ness KK, Ehrhardt MJ, Yasui Y, Duprez D, et al. Major cardiac events for adult survivors of childhood cancer diagnosed between 1970 and 1999: report from the Childhood Cancer Survivor Study cohort. BMJ. 2020;368:l6794.
Cooper TM, Absalon MJ, Alonzo TA, Gerbing RB, Leger KJ, Hirsch BA, et al. Phase I/II study of CPX-351 followed by fludarabine, cytarabine, and granulocyte-colony stimulating factor for children with relapsed acute myeloid leukemia: a report from the Children’s Oncology Group. J Clin Oncol. 2020;38:2170–7.
Getz KD, Sung L, Alonzo TA, Leger KJ, Gerbing RB, Pollard JA, et al. Effect of dexrazoxane on left ventricular systolic function and treatment outcomes in patients with acute myeloid leukemia: a report from the Children’s Oncology Group. J Clin Oncol. 2020;38:2398–406.
Armstrong GT, Oeffinger KC, Chen Y, Kawashima T, Yasui Y, Leisenring W, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31:3673–80.
Dandoy CE, Davies SM, Ahn KW, He Y, Kolb AE, Levine J, et al. Comparison of total body irradiation versus non- total body irradiation containing regimens for de novo acute myeloid leukemia in children. Haematologica. 2020. [Epub ahead of print]
Phillips SM, Padgett LS, Leisenring WM, Stratton KK, Bishop K, Krull KR, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomark Prev. 2015;24:653–63.
Krull KR, Gioia G, Ness KK, Ellenberg L, Recklitis C, Leisenring W, et al. Reliability and validity of the Childhood Cancer Survivor Study Neurocognitive Questionnaire. Cancer. 2008;113:2188–97.
Kenzik KM, Huang IC, Brinkman TM, Baughman B, Ness KK, Shenkman EA, et al. The Childhood Cancer Survivor Study-Neurocognitive Questionnaire (CCSS-NCQ) revised: item response analysis and concurrent validity. Neuropsychology. 2015;29:31–44.
Stefanski KJ, Anixt JS, Goodman P, Bowers K, Leisenring W, Baker KS, et al. Long-Term Neurocognitive and Psychosocial Outcomes After Acute Myeloid Leukemia: A Childhood Cancer Survivor Study Report J Natl Cancer Inst. 2020. In press.
Scott JM, Li N, Liu Q, Yasui Y, Leisenring W, Nathan PC, et al. Association of exercise with mortality in adult survivors of childhood cancer. JAMA Oncol. 2018;4:1352–8.
Tonorezos ES, Ford JS, Wang L, Ness KK, Yasui Y, Leisenring W, et al. Impact of exercise on psychological burden in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2019;125:3059–67.
Howell CR, Krull KR, Partin RE, Kadan-Lottick NS, Robison LL, Hudson MM, et al. Randomized web-based physical activity intervention in adolescent survivors of childhood cancer. Pediatr Blood Cancer. 2018;65:e27216.
Mendoza JA, Baker KS, Moreno MA, Whitlock K, Abbey-Lambertz M, Waite A, et al. A Fitbit and Facebook mHealth intervention for promoting physical activity among adolescent and young adult childhood cancer survivors: a pilot study. Pediatr Blood Cancer. 2017;64:e26660.
Ojha RP, Oancea SC, Ness KK, Lanctot JQ, Srivastava DK, Robison LL, et al. Assessment of potential bias from non-participation in a dynamic clinical cohort of long-term childhood cancer survivors: results from the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2013;60:856–64.
Funding
This study was supported by the National Cancer Institute: Cancer Center Support (CORE) Grant (CA21765) to St. Jude Children’s Research Hospital (PI: Dr. Charles W. Roberts) and U01 CA195547 (MPI: MMH and LLR) and the American Lebanese Syrian Associate Charities (ALSAC), Memphis, TN.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Bhatt, N.S., Baassiri, M.J., Liu, W. et al. Late outcomes in survivors of childhood acute myeloid leukemia: a report from the St. Jude Lifetime Cohort Study. Leukemia 35, 2258–2273 (2021). https://doi.org/10.1038/s41375-021-01134-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-021-01134-3
This article is cited by
-
A scoping review evaluating physical and cognitive functional outcomes in cancer survivors treated with chemotherapy: charting progress since the 2018 NCI think tank on cancer and aging phenotypes
Journal of Cancer Survivorship (2024)
-
Neurocognitive deficits in survivors of childhood acute myeloid leukemia
BMC Pediatrics (2022)