Abstract
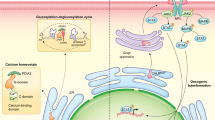
Studies have shown that mutant calreticulin (CALR) constitutively activates the thrombopoietin (TPO) receptor MPL and thus plays a causal role in the development of myeloproliferative neoplasms (MPNs). To further elucidate the molecular mechanism by which mutant CALR promotes MPN development, we studied the subcellular localization of mutant CALR and its importance for the oncogenic properties of mutant CALR. Here, mutant CALR accumulated in the Golgi apparatus, and its entrance into the secretion pathway and capacity to interact with N-glycan were required for its oncogenic capacity via the constitutive activation of MPL. Mutant CALR-dependent MPL activation was resistant to blockade of intracellular protein trafficking, suggesting that MPL is activated before reaching the cell surface. However, removal of MPL from the cell surface with trypsin shut down downstream activation, implying that the surface localization of MPL is required for mutant CALR-dependent activation. Furthermore, we found that mutant CALR and MPL interact on the cell surface. Based on these findings, we propose a model in which mutant CALR induces MPL activation on the cell surface to promote MPN development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med 2013;369:2379–90.
Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med 2013;369:2391–405.
Elf S, Abdelfattah NS, Chen E, Perales-Paton J, Rosen EA, Ko A, et al. Mutant calreticulin requires both its mutant c-terminus and the thrombopoietin receptor for oncogenic transformation. Cancer Discov 2016;6:368–81.
Chachoua I, Pecquet C, El-Khoury M, Nivarthi H, Albu RI, Marty C, et al. Thrombopoietin receptor activation by myeloproliferative neoplasm associated calreticulin mutants. Blood 2016;127:1325–35.
Araki M, Yang Y, Masubuchi N, Hironaka Y, Takei H, Morishita S, et al. Activation of the thrombopoietin receptor by mutant calreticulin in CALR-mutant myeloproliferative neoplasms. Blood 2016;127:1307–16.
Marty C, Chaligne R, Lacout C, Constantinescu SN, Vainchenker W, Villeval JL. Ligand-independent thrombopoietin mutant receptor requires cell surface localization for endogenous activity. J Biol Chem 2009;284:11781–91.
Araki M, Yang Y, Imai M, Mizukami Y, Kihara Y, Sunami Y, et al. Homomultimerization of mutant calreticulin is a prerequisite for MPL binding and activation. Leukemia 2019;33:122–31.
Araki M, Komatsu N. Mutant molecular chaperone activates cytokine receptor as a homomultimer. Oncotarget 2018;9:35201–2.
Takei H, Edahiro Y, Mano S, Masubuchi N, Mizukami Y, Imai M, et al. Skewed megakaryopoiesis in human induced pluripotent stem cell-derived haematopoietic progenitor cells harbouring calreticulin mutations. Br J Haematol 2018;181:791–802.
Sunami Y, Araki M, Hironaka Y, Morishita S, Kobayashi M, Liew EL, et al. Inhibition of the NAD-dependent protein deacetylase SIRT2 induces granulocytic differentiation in human leukemia cells. PloS ONE 2013;8:e57633.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012;9:671–5.
Komatsu N, Kunitama M, Yamada M, Hagiwara T, Kato T, Miyazaki H, et al. Establishment and characterization of the thrombopoietin-dependent megakaryocytic cell line, UT-7/TPO. Blood 1996;87:4552–60.
Michalak M, Groenendyk J, Szabo E, Gold LI, Opas M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem J 2009;417:651–66.
Pronier E, Cifani P, Merlinsky TR, Berman KB, Somasundara AVH, Rampal RK, et al. Targeting the CALR interactome in myeloproliferative neoplasms. JCI Insight 2018;3:122703. pii
Elf S, Abdelfattah NS, Baral AJ, Beeson D, Rivera JF, Ko A, et al. Defining the requirements for the pathogenic interaction between mutant calreticulin and MPL in MPN. Blood 2018;131:782–6.
Kapoor M, Ellgaard L, Gopalakrishnapai J, Schirra C, Gemma E, Oscarson S, et al. Mutational analysis provides molecular insight into the carbohydrate-binding region of calreticulin: pivotal roles of tyrosine-109 and aspartate-135 in carbohydrate recognition. Biochemistry 2004;43:97–106.
Dahlen DD, Broudy VC, Drachman JG. Internalization of the thrombopoietin receptor is regulated by 2 cytoplasmic motifs. Blood 2003;102:102–8.
Pecquet C, Balligand T, Chachoua I, Roy A, Vertenoeil G, Colau D, et al. Secreted mutant calreticulins as rogue cytokines trigger thrombopoietin receptor activation specifically in CALR mutated cells: perspectives for MPN therapy. Blood 2018;132:4.
Pecquet C, Chachoua I, Roy A, Balligand T, Vertenoeil G, Leroy E, et al. Calreticulin mutants as oncogenic rogue chaperones for TpoR and traffic-defective pathogenic TpoR mutants. Blood 2019;133:2669–81.
Acknowledgements
This work was funded in part by the MEXT-Supported Program for the Strategic Research Foundation at Private Universities; MEXT’s Promotion Plan for the Platform of Human Resource Development for Cancer Project; the JSPS KAKENHI Grants #15K15368, #16K09859, #17K16195, #17H04211, #18K08372, #18K16126, # 18K16127, #18K16098, and #19K08848; grants from the Takeda Science Foundation, the SENSHIN Medical Research Foundation and the Japan Leukemia Research Fund; and personal research funds from Haruhito Hosoya, Yuki Ishibe, Yoshitsugu Katayose, Mikio Kato, Masae Kunitama, Kumie Kurita, Masahiro Nakamura, Kaori Taki, Tetsuya Takahashi, Kanji Shishido, and Hiroaki Yamakawa. The funders had no role in the preparation of the manuscript. We are grateful to Shing Leng Chan for her critical reading of the manuscript and to Soichiro Kakuta and Kazuhito Naka for their productive suggestions. We would like to thank other members of the Department of Hematology for supporting this study. We would also like to acknowledge the Laboratory of Molecular and Biochemical Research, the Laboratory of Morphological Analysis and Imaging, and the Division of Cell Biology in the Research Support Center of the Juntendo University Graduate School of Medicine.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: NM, MA, and NK. Performed the experiments: NM, MA, YY, EH, MI, YE, YH, YM, YK, HT and MN. Analyzed the data: NM, MA, YY, EH, MI and YE. Contributed reagents/materials/analysis tools: MK, AO and NK. Wrote the paper: NM, MA and NK.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Masubuchi, N., Araki, M., Yang, Y. et al. Mutant calreticulin interacts with MPL in the secretion pathway for activation on the cell surface. Leukemia 34, 499–509 (2020). https://doi.org/10.1038/s41375-019-0564-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-019-0564-z
This article is cited by
-
Oncogenic CALR mutant C-terminus mediates dual binding to the thrombopoietin receptor triggering complex dimerization and activation
Nature Communications (2023)
-
A marine sponge-derived lectin reveals hidden pathway for thrombopoietin receptor activation
Nature Communications (2022)
-
Essential thrombocythaemia progression to the fibrotic phase is associated with a decrease in JAK2 and PDL1 levels
Annals of Hematology (2022)
-
Myeloproliferative neoplasm-driving Calr frameshift promotes the development of pulmonary hypertension in mice
Journal of Hematology & Oncology (2021)
-
MPL overexpression induces a high level of mutant-CALR/MPL complex: a novel mechanism of ruxolitinib resistance in myeloproliferative neoplasms with CALR mutations
International Journal of Hematology (2021)