Abstract
Nasal natural killer/T-cell lymphoma (NNKTL) is closely associated with Epstein-Barr virus (EBV) and is characterized by poor prognosis, resulting from rapid progression of lesions in the affected organs. Recent data have shown that NNKTL is associated with the aberrant expression of cyclin-dependent kinase 1 (CDK1) and its downstream target survivin, but little is known about the functional roles of CDK1 and survivin in NNKTL. In the current study, we show that knockdown of the EBV-encoded oncoprotein latent membrane protein 1 (LMP1) induces downregulation of CDK1 and survivin in NNKTL cells. Immunohistochemistry detected CDK1 and survivin expression in LMP1-positive cells of NNKTL biopsy specimens. Inhibition of CDK1 and survivin in NNKTL cells with several inhibitors led to a dose-dependent decrease in cell proliferation. In addition, the Sp1 inhibitor mithramycin, which can downregulate both CDK1 and survivin, significantly suppressed the growth of established NNKTL in a murine xenograft model. Our results suggest that LMP1 upregulation of CDK1 and survivin may be essential for NNKTL progression. Furthermore, targeting CDK1 and survivin with Sp1 inhibitors such as mithramycin may be an effective approach to treat NNKTL, which is considered to be a treatment-refractory lymphoma.
Similar content being viewed by others
Introduction
Nasal natural killer/T-cell lymphoma (NNKTL) shows distinct epidemiological, clinical, histological, and etiological features compared to other malignant lymphomas, and is more common in Asian countries than in the United States and Europe [1]. NNKTL is characterized by progressive necrotic lesions with infiltration of many inflammatory cells, such as lymphocytes, monocytes, and macrophages, mainly in the nasal or oral cavity, and manifests poor prognosis resulting from rapid lesion progression in the affected organs [1,2,3,4]. Our group was the first to establish that Epstein-Barr virus (EBV) DNA, EBV-oncogenic proteins, and clonotypic EBV genomes are present in this lymphoma, indicating that EBV might play an important role in lymphomagenesis [2, 5, 6]. With regard to the expression of EBV-oncogenic proteins and mRNAs, EBV nuclear antigen 1 (EBNA1) is expressed in NNKTL, but other EBNAs are not [2, 7]. Although latent membrane protein 1 (LMP1) mRNA is expressed in all tumors, the protein is found to be expressed in only about 50% of patients because of highly-methylated LMP coding sequences [2, 6,7,8]. Therefore, NNKTL is categorized as representing an EBV infection with type II latency.
LMP1 is known to be pivotal in the EBV-mediated transformation of B cells [9], and is regarded as an oncoprotein because it can induce the transformation of rodent fibroblast cell lines in vitro [10]. Furthermore, these transformed cell lines are tumorigenic in nude mice [10]. It is therefore of great interest to clarify the involvement of LMP1 in the tumorigenesis of NNKTL. We previously showed that NNKTL cells produce several cytokines and signaling molecules that may be regulated by LMP1, such as interleukin-9 (IL-9), IL-10, interferon-γ-inducible protein 10, chemokine (C-C motif) ligand 17 (CCL17), CCL22, and soluble intercellular adhesion molecule 1, and that these play significant roles in the proliferation and invasion of tumor cells in an autocrine manner [11,12,13,14,15]. We further showed that LMP1 can induce upregulation of CD70 and downregulation of micro-RNA-15a, resulting in proliferation [16, 17]. Thus, the roles of LMP1 in NNKTL are gradually being elucidated.
Cyclin-dependent kinase 1 (CDK1), along with other members of this family, controls cell cycle progression and transcription and is regulated by the presence of cyclin partners [18]. CDK1 is reported to partner with cyclins A and B to promote G2/M phase progression [19]. Survivin is an anti-apoptotic factor involved in regulating apoptosis and mitotic spindle functions [20] and is phosphorylated at Thr34 by the complex of CDK1-cyclin B. In the absence of Thr34 phosphorylation, survivin is rapidly degraded, resulting in the disruption of survivin-caspase 9 complexes and leading to an increase in caspase 9-dependent apoptosis in M phase [21, 22]. We previously found that survivin is highly expressed in EBV-positive post-transplant diffuse large B-cell lymphoma, and that treatment with CDK or survivin inhibitors leads to the death of EBV-transformed B cells [23]. Recent gene expression profiling using formalin-fixed, paraffin-embedded (FFPE) tissues from NNKTL patients has shown that NNKTL is associated with the aberrant expression of CDK1 and survivin [24]. However, little is known about the functional roles of CDK1 and survivin and their association with LMP1 in NNKTL.
Here, we investigated the involvement of LMP1 in the regulation of CDK1 expression and its downstream target survivin, and the influence of inhibitors of these proteins on proliferation in NNKTL. We demonstrate that LMP1 mediates the expression of CDK1 and survivin in NNKTL cell lines. In addition, inhibitors of CDK1 and survivin, such as mithramycin and terameprocol, reduced the proliferation of NNKTL cells via the induction of apoptosis and cell cycle arrest. Furthermore, mithramycin induced anti-tumor effects in an NNKTL xenograft mouse model. These results suggest that CDK1 and survivin over-expressed by LMP1 are potential therapeutic targets for the treatment of NNKTL.
Materials and methods
Ethics statement
This study was conducted in accordance with the principles expressed in the Declaration of Helsinki, and was approved by the Institutional Review Board of Asahikawa Medical University. All subjects provided written informed consent for the collection of samples and subsequent analysis.
Patients
Twelve NNKTL patients, 11 male and 1 female, 21–79 years of age with a median age of 64.5 years, were analyzed in this study and were diagnosed according to the World Health Organization classification of hematologic malignancies [25] at the Department of Otolaryngology-Head and Neck Surgery, Asahikawa Medical University (Asahikawa, Japan) between 2003 and 2018.
Cell lines, reagents, and cell treatments
Two EBV and LMP1-positive NNKTL cell lines, SNK-6 and SNT-8 [26], were kindly provided by Dr. Norio Shimizu (Tokyo Medical and Dental University, Tokyo, Japan). KHYG-1 established from patients with NK-cell leukemia is an EBV-negative NK-cell line and was purchased from the Health Science Research Resources Bank (Osaka, Japan). The EBV-negative T-cell leukemia cell line Jurkat was purchased from the American Type Culture Collection (Manassas, VA). SNK-6 and SNT-8 were cultured in RPMI1640 supplemented with 10% human serum, penicillin (100 U/mL), streptomycin (100 g/mL), and recombinant human IL-2 (700 U/mL). KHYG-1 was cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/mL), streptomycin (100 g/mL), and recombinant human IL-2 (100 U/mL). Jurkat was cultured in RPMI 1640 supplemented with 10% FBS, penicillin (100 U/mL), and streptomycin (100 g/mL). For treatment, the cells were split to a concentration of 3 × 105 cells/mL 16 h before treatment. Increasing concentrations of PD0332991 (Selleck Chemicals, Houston, TX), flavopiridol (Selleck Chemicals), YM155 (Selleck Chemicals), terameprocol (Sigma-Aldrich, St. Louis, MO), and mithramycin (Sigma-Aldrich) at 10 mM dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich) as a stock solution, or the corresponding maximum volume of DMSO as a control, were added to the cells. Cells were collected after 24, 48, and/or 72 h and subjected to analyses of mRNA and protein expression, proliferation, and cell cycle.
RNA extraction and quantitative real-time polymerase chain reaction (qPCR)
Total RNA was extracted using an RNeasy Mini Kit (Qiagen, Hilden, Germany). After DNase treatment (DNase free; Applied Biosystems, Foster City, CA), 1 μg of total RNA was used as a template for reverse transcription using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). qPCR for human and EBV mRNA was performed using specific primers and probes for LMP1 as described previously [27], CDK1, and survivin (Applied Biosystems). All reactions were performed on a real-time PCR machine (7900HT; Applied Biosystems) with TaqMan Gene Expression Master Mix (Applied Biosystems). The relative gene expression was calculated for each gene of interest using the ΔΔCT method, where CT values were normalized to the housekeeping gene hydroxymethylbilane synthase (HMBS) [28].
Western blot analysis
Cell extracts were prepared in Laemmli buffer (4% SDS, 20% glycerol, and 120 mM Tris, pH 6.8). Electrophoresis and transfer to a membrane were performed using the NuPAGE system (Invitrogen, Carlsbad, CA). Antibodies against LMP1 (CS1-4; Santa Cruz Biotechnology, Santa Cruz, CA), CDK1 (Cell Signaling Technology, Danvers, MA), survivin (71G4B7; Cell Signaling Technology), poly ADP-ribose polymerase (PARP; Cell Signaling Technology), β-actin (Sigma-Aldrich), and α-tubulin (Sigma-Aldrich) were used for immunoblots.
MTS assay and cell cycle analysis
MTS assays were performed using the CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI) as described previously [11, 13, 16]. For cell cycle analysis, cells were harvested, washed in cold phosphate-buffered saline (PBS), and fixed in 70% ethanol. DNA was stained by incubating the cells in PBS containing propidium iodide (50 mg/mL, Sigma-Aldrich) and RNase A (100 mg/mL, Sigma-Aldrich) for 30 min at 37 °C. Cell cycle data were collected using the FACSCanto II (BD Biosciences, San Jose, CA) and analyzed using FlowJo software.
RNA interference for LMP1
Transfection of siRNA was carried out using the Neon Transfection System (Invitrogen) as described previously [17]. Briefly, 2.5 × 105 cells suspended in 10 µL of buffer containing 50 nM siRNAs were electroporated using three pulses of 1500 V for 10 ms. After electroporation, cells were resuspended in fresh media and analyzed after 48 h. Silencer Select siRNA against LMP1 was synthesized by Applied Biosystems, with the siRNA target sequence to LMP1 mRNA being aacugguggacucuauugguu [29]. Silencer Select Negative Control #1 siRNA was also purchased from Applied Biosystems.
Immunohistochemistry and in situ hybridization
FFPE samples were prepared from pretreated biopsy tissues of NNKTL patients or from subcutaneous tumors of mice transplanted with SNK-6 cells and were cut into 4-µm thick sections. We used anti-CD56 (1:50, Novocastra, Newcastle Upon Tyne, UK), anti-LMP1 (CS1-4, 1:100, Dako, Glostrup, Denmark), anti-CDK1 (E161, 1:500, Abcam plc, Cambridge, UK), and anti-survivin (71G4B7, 1:400, Cell Signaling Technology) as the primary antibodies. The Envision HRP System (Dako) was used for visualization of the signals. For antigen retrieval, slides were treated with Target Retrieval Solution pH 9 (Dako, for use with CD56 and LMP1 antibodies) or 10 mmol/L citric acid buffer at pH 6.0 (for use with CDK1 and survivin antibodies) using a Decloaking Chamber (Biocare Medical, Pacheco, CA) for 10 min at 110 °C. Serial sections were used for CD56, LMP1, CDK1, and survivin staining. A case was considered as CDK1 or survivin positive if ≥10% of the CD56-positive tumor cells were also CDK1 or survivin positive as previously described by other groups [30,31,32]. EBV-encoded small RNA (EBER) in FFPE tissue sections was detected by in situ hybridization (ISH) as previously described [2]. Two-color immunohistological studies were performed on 4-μm-thick FFPE tissue sections using the EnVision™ G|2 Doublestain System (Dako). Anti-LMP1 (1:100), anti-CDK1 (1:50), and anti-survivin (1:100) antibodies were used. Prior to two-color staining, slides were placed in a 10 mmol/L citric acid buffer at pH 6.0 and underwent antigen retrieval for 15 min at 750 W in a microwave oven.
Mice
Six-week-old female NOD/Shi-scid/IL-2Rγnull (NOG) mice were obtained from the Central Institute for Experimental Animals (Kawasaki, Japan) and were maintained under specific pathogen-free conditions in the Animal Laboratory of the Center for Advanced Research and Education, Asahikawa Medical University (Asahikawa, Japan). All animal experiments were approved by the Institutional Animal Care and Use Committee of Asahikawa Medical University.
Therapeutic protocols and evaluation of anti-tumor effects
NOG mice were injected subcutaneously with 5 × 106 SNK-6 cells in a shaved flank as described previously [33]. Mithramycin was dissolved in PBS and administrated intraperitoneally at 0.2 mg/kg, three times per week, starting when tumor sizes reached approximately 25 mm2 (day 0). Control mice received an equal volume of PBS. Tumor growth was monitored every 3–4 days in individually tagged mice by measuring two opposing diameters with a set of calipers. Mice were euthanatized and tumors were weighed when the tumor area of the control group on average reached approximately 400 mm2 (day 25).
Statistical analyses
An unpaired Student’s t-test was used to compare tumor weights between mithramycin-treated and control mice. Differences in tumor sizes between populations over time were analyzed for significance using a two-way ANOVA. P < 0.05 was considered statistically significant. All analyses and graphics were performed using GraphPad Prism 5 (GraphPad Software).
Results
Knockdown of LMP1 in NNKTL cells induces downregulation of CDK1 plus survivin and apoptosis
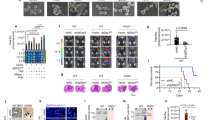
To investigate whether LMP1 is involved in the expression of CDK1 and survivin in NNKTL, we first performed LMP1 siRNA transfection in the SNK-6 and SNT-8 cell lines, which were established from human primary NNKTL lesions and are positive for LMP1 [26]. As shown in Fig. 1a, qPCR showed that the expression of LMP1 was reduced in transfected SNK-6 and SNT-8 cells. The expression of CDK1 and survivin was also downregulated in both cell lines, concomitant with the knockdown of LMP1. Western blot confirmed that CDK1 and survivin expression were decreased in the LMP1 siRNA-transfected cells (Fig. 1b). Concomitantly, the cleavage of PARP, which repairs damaged DNA in malignant cells and is inactivated by caspase cleavage, was observed in both lines (Fig. 1b). We also examined whether the direct knockdown of CDK1 or survivin using siRNA induces the cleavage of PARP to confirm that CDK1 and survivin play significant roles in the control of apoptosis in NNKTL cells. In SNK-6 and SNT-8 cells transfected with CDK1 or survivin siRNA, the increased cleavage of PARP was detected concurrently with the reduction of CDK1 or survivin protein (Supplementary Figure S1). To strengthen our results regarding the regulation of CDK1 and survivin expression by LMP1, we further examined the expression of both molecules in NNKTL cells cultured with or without IL-2, because IL-2 is important to maintain the expression of LMP1 in NNKTL cells [12]. As shown in Supplementary Figure S2a, the expression of LMP1 mRNA was reduced in IL-2-starved SNK-6 cells after 24 and 48 h cultures compared to IL-2-treated SNK-6 cells, whereas the expression of EBNA-1 mRNA did not change. Western blot analysis showed that the expression of LMP1 protein was also decreased in SNK-6 cells cultured without IL-2, concomitant with the cleavage of PARP (Supplementary Figure S2b). Under starvation of IL-2, which indirectly resulted in decreased expression of LMP1, the mRNA and protein expression of CDK1 and survivin were expectedly downregulated in NNKTL cells (Supplementary Figures S2c and d). Moreover, we performed gene expression profiling using this model to examine the expression of other CDK and baculoviral IAP repeat-containing (BIRC) genes. As shown in Supplementary Figure S2e, using a 1.5-fold change and P < 0.05 as cut-offs, only 5 genes including CDK1 and survivin (CDK1, CDK2, BIRC3, XIAP, and survivin) were upregulated in IL-2-treated cells compared to IL-2-starved cells. These results indicate that LMP1 plays an important role in the regulation of CDK1 and survivin expression and anti-apoptosis in NNKTL tumor cells.
Knockdown of LMP1 induces downregulation of CDK1 and survivin expression and apoptosis in NNKTL cells. a qPCR analysis of LMP1, CDK1, and survivin expression in SNK-6 and SNT-8 cells 48 h after transfection with either negative control or LMP1 siRNA. Expression of mRNA is shown relative to that of control cells. Data represent the mean ± SEM of three independent experiments. b Western blot analysis of PARP, LMP1, CDK1, and survivin expression in the same cells used in (a), with β-actin used as a loading control
LMP1-positive lymphoma cells express CDK1 and survivin in NNKTL tissues
We next performed immunohistochemical staining to examine whether CDK1 and survivin are expressed in lymphoma cells using biopsy tissues from 12 NNKTL patients. Atypical lymphoid cells infiltrated the nasal mucosa (Fig. 2a) and were positive for EBER (Fig. 2b) in all samples. In addition, CD56 was also expressed in all 12 patients (Fig. 2c). Eight of 12 samples (67%) were positive for LMP1 (Fig. 2d). Staining using serial sections showed that CDK1 and survivin were expressed in 6 (75%) and 7 (88%) of 8 LMP1-positive samples respectively and that CD56 and LMP1-positive lymphoma cells were preferentially localized at CDK1 and survivin-expressing areas (Fig. 2c–f). To confirm that LMP1-positive tumor cells expressed CDK1 and survivin, two-color immunohistochemical staining for LMP1 and CDK1 or survivin was performed, and as a result, LMP1-positive cells co-expressed CDK1 (Fig. 2g) and survivin (Fig. 2h). We further examined the expression of CDK1 and survivin in 4 LMP1-negative samples using serial sections (Supplementary Figure S3). CDK1 and survivin were also expressed in 3 (75%) and 3 (75%) of 4 LMP1-negative samples, respectively. However, the percentage of CDK1 or survivin-positive cells in tumor cells was less than 50% in LMP1-negative samples (Supplementary Figure S3), while >50% of the tumor cells were positive for CDK1 or survivin in LMP1-positive samples (Fig. 2c–h). These results suggest that LMP1 might upregulate the expression of CDK1 and survivin not only in cultured cell lines, but also in the lymphoma cells of NNKTL patients.
Expression of CDK1 and survivin in LMP1-positive lymphoma cells of NNKTL biopsy tissues. Representative immunohistological features of FFPE samples are shown. a The nasal mucosa is infiltrated by atypical lymphoid cells (H&E staining). b ISH for EBER. Nuclei of EBER-positive cells are stained brown. c Staining for CD56. d Staining for LMP1. e Staining for CDK1. f Staining for survivin. Serial sections were used for c–f. Scale bar in a–f is 100 μm. g Two-color immunohistochemistry of CDK1 (brown) and LMP1 (red). h Two-color immunohistochemistry of survivin (brown) and LMP1 (red). In g and h, nuclei are mainly stained brown in CDK1- or survivin-positive cells, while the cytoplasm is mainly stained red in LMP1-positive cells. Scale bar in g and h is 25 μm
Sp1-inhibitors mithramycin and terameprocol suppress NNKTL cell proliferation
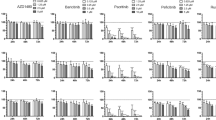
We postulated that inhibiting CDK1 and survivin in NNKTL cells might be effective in suppressing proliferation. Mithramycin and terameprocol are competitive inhibitors of the Sp1 transcription factor, which mediates CDK1 and survivin expression [34]. We thus analyzed the proliferation of SNK-6 and SNT-8 cells treated with mithramycin or terameprocol by MTS assay. Both agents dose-dependently suppressed the proliferation of SNK-6 (Fig. 3a, b) and SNT-8 (Fig. 3c, d) to a significant extent. In contrast, the pan-CDK inhibitor flavopiridol (Fig. 3e) and the survivin inhibitor YM155 (Fig. 3f) suppressed the proliferation of SNK-6 moderately. The CDK4/6 inhibitor PD0332991 did not suppress proliferation (Fig. 3g). These results indicate that the Sp1-inhibitors mithramycin and terameprocol efficiently suppress the growth of NNKTL cells. We further examined the effect of mithramycin and terameprocol against the EBV-negative T-cell line Jurkat and the NK-cell line KHYG-1 to compare with EBV-positive NNKTL cell lines. Both agents also suppressed the proliferation of EBV-negative cell lines (Fig. 3h–k). We found no significant differences between NNKTL cell lines and EBV-negative T and NK-cell lines treated with mithramycin (Fig. 3h, j), while terameprocol showed a weak suppressive effect against EBV-negative cell lines compared with NNKTL cell lines (Fig. 3i, k).
Sp1-inhibitors mithramycin and terameprocol suppress proliferation of NNKTL cells. SNK-6 cells were cultured with various concentrations of mithramycin (inhibitor of Sp1, a), terameprocol (inhibitor of Sp1, b), flavopiridol (inhibitor of pan-CDK, e), YM155 (inhibitor of survivin, f), or PD0332991 (inhibitor of CDK4 and CDK6, g) for 72 h. SNT-8 cells were cultured in the presence of various doses of mithramycin (c) or terameprocol (d) for 72 h. EBV-negative cell lines, KHYG-1 and Jurkat, were cultured with various concentrations of mithramycin (h, j) or terameprocol (i, k) for 72 h. The number of viable cells was measured by MTS assay. Presented data (mean ± SEM) are relative to untreated (DMSO only) controls, and all assessments were carried out in triplicate
Treatment with Sp1-inhibitors decreases CDK1 and survivin expression and induces apoptosis and G2/M cell cycle arrest in NNKTL cells
To confirm that Sp1-inhibitors indeed inhibit CDK1 and survivin in NNKTL cells, we analyzed CDK1 and survivin expression in mithramycin- or terameprocol-treated SNK-6 cells. As shown in Fig. 4a, qPCR indicated a time-dependent decrease in the expression of CDK1 and survivin after treatment with mithramycin or terameprocol. Western blot analysis further confirmed these data and showed that the expression of CDK1 and survivin was dose-dependently decreased by a 48-h incubation with mithramycin or terameprocol (Fig. 4b). A dose-dependent increase in the cleavage of PARP was also observed, concomitant with the inhibition of CDK1 and survivin (Fig. 4b), suggesting that mithramycin and terameprocol induce apoptosis in SNK-6 cells. Additionally, we performed flow cytometry cell cycle analysis of SNK-6 cells treated with or without mithramycin. As shown in Fig. 4c, mithramycin decreased the G1 phase cell population and increased the G2/M phase cell population. This result indicates that G2/M cell cycle arrest is induced through the inhibition of CDK1 and survivin by mithramycin, consistent with the fact that CDK1 and survivin have been reported to be expressed and function mainly in the G2/M phase of the cell cycle.
Treatment with Sp1 inhibitors decreases CDK1 and survivin expression and induces apoptosis and G2/M cell cycle arrest in NNKTL cells. a SNK-6 cells were treated with 100 nM mithramycin or 100 µM terameprocol for 24, 48, and 72 h. CDK1 and survivin mRNA expression were analyzed by qPCR, with data (mean ± SEM) shown relative to untreated (DMSO only) controls. b SNK-6 cells were cultured with various concentrations of mithramycin (100, 330, and 1000 nM) or terameprocol (1, 10, and 100 µM) for 48 h. Protein levels of PARP, CDK1, and survivin were analyzed by western blot, with β-actin used as a loading control. c Cell cycle analysis of SNK-6 cells treated with vehicle (untreated control) or 100 nM mithramycin for 48 h was performed by flow cytometry. Black arrows indicate G1 phase and white arrows indicate G2/M phase
Mithramycin shows anti-tumor effects in a murine xenograft model of NNKTL
We then extended our investigation to an in vivo murine xenograft model, using NOG mice inoculated subcutaneously with SNK-6, to ascertain the importance of our in vitro findings. To examine the expression of CDK1 and survivin, we then performed immunohistochemical staining using FFPE sections from these tumors. Tumor cells showed positive nuclear and cytoplasmic staining for CDK1 (Fig. 5a), while the expression of survivin was detected in the nucleus (Fig. 5b). Almost all tumor cells abundantly expressed LMP1 (Fig. 5c), suggesting that LMP1 might be associated with the upregulation of CDK1 and survivin, not only in cultured SNK-6 cells, but also in SNK-6 tumors grown in vivo.
Expression of LMP1, CDK1, and survivin is detected in tumor tissues from a murine xenograft model inoculated with SNK-6 cells. NOG mice were injected subcutaneously with 2 × 106 SNK-6 cells in a shaved flank. Thirty days later, mice were sacrificed and the tumors collected, fixed with formalin, embedded in paraffin, and cut into 4-µm thick sections. Representative immunohistological features of tumor samples are shown. a Staining for CDK1. b Staining for survivin. c Staining for LMP1. Scale bar, 100 µm
Finally, we evaluated the therapeutic efficacy of mithramycin in this subcutaneous xenograft model. After tumor sizes reached approximately 25 mm2, mice were treated either with vehicle (PBS) or mithramycin three times per week. Treatment with mithramycin significantly reduced mean tumor volume (Fig. 6a, P = 0.0254; Fig. 6b, c) and weight (Fig. 6d; P = 0.0186) compared to PBS controls. We further assessed whether apoptosis was induced in tumors treated with mithramycin using immunohistochemistry for the apoptotic markers, cleaved caspase 3 and PARP (Supplementary Figure S4). More tumor cells were stained positively for cleaved caspase 3 and PARP in tumors treated with mithramycin compared to tumors with no treatment, suggesting that the increased apoptosis could contribute to decreased tumor growth in the group treated with mithramycin. These results indicate that mithramycin might be a good candidate for the treatment of NNKTL.
Mithramycin shows anti-tumor effects in a murine xenograft model inoculated with SNK-6 cells. The therapeutic effect of mithramycin in NOG mice xenografted with 5 × 106 SNK-6 cells in a shaved flank is shown. Mice (4 per group) were intraperitoneally treated with either vehicle (PBS) or 0.2 mg/kg/day mithramycin, three times per week, when tumor size reached approximately 25 mm2 (day 0). a Treatment with mithramycin significantly reduced average tumor size compared to no treatment (PBS controls). Data represent the mean ± SD for each group of mice; significance was assessed by two-way ANOVA (*P < 0.05). b, c Representative images of tumors treated with or without mithramycin at day 25. d The weights of the tumors were calculated on day 25. Data represent mean weight ± SD; significance was assessed by unpaired Student’s t-test (*P < 0.05)
Discussion
In the present study, we aimed to establish the functional roles of CDK1 and survivin in NNKTL. We found that (1) knockdown of LMP1 results in downregulation of CDK1 and survivin and apoptosis in NNKTL cells; (2) LMP1-positive lymphoma cells from primary NNKTL tissues abundantly express CDK1 and survivin; (3) the Sp1-inhibitors mithramycin and terameprocol strongly suppress the proliferation of NNKTL cells; and (4) mithramycin exhibits anti-tumor effects in a NNKTL murine xenograft model. Thus, the upregulation of CDK1 and survivin by LMP1 may be essential for NNKTL progression, and targeting CDK1 and survivin with Sp1-inhibitors such as mithramycin could therefore represent a potential novel therapy for the notoriously treatment-refractory NNKTL.
To the best of our knowledge, this is the first report to describe that the direct knockdown of LMP1 induces down-regulation of CDK1 expression in NNKTL cells. Suzuki et al. [35] recently reported that the heat shock protein 90 inhibitor BIIB021 decreases the expression of LMP1 in EBV-positive T and NK lymphoma cell lines. Interestingly, BIIB021 also downregulated the protein levels of CDK1, indicating that the decreased expression of LMP1 by BIIB021 might induce downregulation of CDK1 expression. In regard to the regulation of survivin by LMP1 in EBV-related tumorigenesis, Sun et al. [36] reported that survivin expression was induced by LMP1 in NNKTL cells. Several groups have also indicated that LMP1 can induce the expression of survivin in nasopharyngeal carcinoma [37,38,39]. In immunohistochemical analysis using tissue samples from NNKTL patients, we found that the percentage of CDK1 or survivin-positive cells among tumor cells was more than 50% in LMP1-positive samples, whereas 10~50% of the tumor cells were positive for CDK1 or survivin in LMP1-negative ones, suggesting that LMP1-positive cases might express higher levels of CDK1 and survivin than LMP1-negative cases in NNKTL, although further study with a greater sample size is required to evaluate this. Our results and the data of other researchers therefore indicate that LMP1 is closely involved in the high expression of CDK1 and survivin in NNKTL and nasopharyngeal carcinoma. On the other hand, Diamantopoulos et al. [40] found that in patients with leukemic low-grade B-cell lymphoma, survivin mRNA expression was significantly higher in LMP1-negative patients than in LMP1-positive patients, although the expression of protein levels in patient samples and in vitro analysis using cell lines to confirm this result were not examined, suggesting the possibility that the regulation of survivin by LMP1 might differ among EBV-associated malignancies. In the present study, we also confirmed that knockdown of LMP1 indeed induces downregulation of survivin in NNKTL and results in the promotion of apoptosis. However, it is possible that LMP1 indirectly upregulates the expression of survivin through the induction of CDK1, because survivin has been reported to be a target of CDK1 and cyclin B phosphorylation [21, 22]. Indeed, using qPCR, we showed that the expression of CDK1 was more decreased than the expression of survivin in LMP1 siRNA-transfected NNKTL cells (Fig. 1a). Thus, while the expression of CDK1 may be directly regulated by LMP1, survivin expression may be indirectly-regulated. Consistent with this, the pan-CDK inhibitor flavopiridol was more effective than the survivin inhibitor YM155 in reducing the viability of NNKTL cells, although the CDK4/6 inhibitor PD0332991 was not effective (Fig. 3e–g). Thus, targeting CDKs other than CDK4 and 6, particularly CDK1, might be a more effective approach to control NNKTL cell growth than just targeting survivin.
In regard to the mechanism of the upregulation of CDK1 by LMP1, previous studies have indicated that CDK1 is activated by extracellular signal-regulated kinase (ERK) signaling [41], and that LMP1 mediates ERK activation [42]. This suggests that CDK1 upregulation by LMP1 in NNKTL may be mediated via a mitogen-activated protein kinase signaling pathway that includes ERK. Notably, a recent study reported that survivin is upregulated by nuclear factor-kappa B and phosphoinositide 3-kinase/Akt signaling pathways activated by LMP1 [36], although as mentioned above, CDK1 induced by LMP1 might also partially upregulate survivin. Nevertheless, the precise signaling pathways mediating the regulation of CDK1 and survivin expression by LMP1 in NNKTL remain to be explored.
Mithramycin and terameprocol are known to inhibit the Sp1 transcription factor, blocking cell cycle progression by inhibiting the expression of CDK1 and simultaneously promoting apoptosis by inhibiting the expression of survivin [34, 43]. In NNKTL cell lines treated with Sp1 inhibitors, we found that mithramycin suppressed proliferation at a lower dose compared to terameprocol (Fig. 3a–d). Moreover, CDK1 and survivin suppression and cleavage of PARP were detected in SNK-6 cells treated with 100 nM mithramycin, while terameprocol induced these changes at a concentration of 100 μM (Fig. 4b). These results suggest that mithramycin might be better for the treatment of NNKTL than terameprocol, because mithramycin could induce anti-tumor effects at lower doses. To evaluate the anti-tumor effect of mithramycin for NNKTL in vivo, we used NOG mice inoculated subcutaneously with SNK-6 cells, and found that mithramycin treatment significantly suppressed tumor growth via the induction of apoptosis. On the other hand, mithramycin and terameprocol also suppressed the in vitro proliferation of EBV-negative T and NK-cell lines (Fig. 3h–k). Interestingly, the cell growth inhibitory activity of terameprocol was stronger against NNKTL cells than against EBV-negative T and NK cells. Thus, it is possible that mithramycin and terameprocol inhibit the proliferation of EBV-negative T and NK cells via a mechanism different from the suppression of CDK1 and survivin upregulated by LMP1 in EBV-positive NNKTL cells, and that terameprocol has a selective anti-tumor effect against EBV-positive T and NK-cell malignancies compared to EBV-negative ones. Future studies will be required to clarify whether there is a difference in drug specificity against T and NK cells with or without EBV infection between mithramycin and terameprocol. Although we did not evaluate the anti-tumor effect of terameprocol in the present study, Sun et al. [36] have recently shown that terameprocol inhibits the growth of tumors in nude mice subcutaneously inoculated with SNT-8 cells transfected with a plasmid overexpressing LMP1 (enhancing the expression of survivin). In view of the suppressive effect of CDK and survivin inhibitors in vitro and in vivo on the proliferation and survival of NNKTL cells documented by us and other investigators [36], it is tempting to propose clinical studies of these agents in NNKTL patients. Mithramycin, which is an aureolic acid-type polyketide isolated from Streptomyces, was originally evaluated as a chemotherapeutic agent in patients with a variety of malignancies during the 1960s [44,45,46] and is an FDA-approved chemotherapeutic agent (NSC 24559) that is effective against testicular carcinoma and chronic myeloid leukemia. YM155, flavopiridol, and terameprocol have also shown a good pharmacological profile in several clinical trials [47,48,49,50,51], and flavopiridol is currently in a phase II clinical trial for relapsed or refractory acute myeloid leukemia (NCT02520011). Thus, CDK and survivin inhibitors such as mithramycin and flavopiridol could be readily developed for clinical use as therapeutic agents in NNKTL. Although the prognosis of patients with early-stage NNKTL has been gradually improving, as shown in previous reports by several groups including our own [52, 53], overall prognosis remains quite poor, especially in the late stages and during disease recurrence. We believe that our findings provide preclinical evidence of the potential of CDK and survivin inhibitors, especially mithramycin, as novel therapeutic strategies for the treatment of NNKTL.
References
Harabuchi Y, Takahara M, Kishibe K, Moriai S, Nagato T, Ishii H. Nasal natural killer (NK)/T-cell lymphoma: clinical, histological, virological, and genetic features. Int J Clin Oncol. 2009;14:181–90.
Harabuchi Y, Imai S, Wakashima J, Hirao M, Kataura A, Osato T, et al. Nasal T-cell lymphoma causally associated with Epstein-Barr virus: clinicopathologic, phenotypic, and genotypic studies. Cancer. 1996;77:2137–49.
Jaffe ES, Chan JK, Su IJ, Frizzera G, Mori S, Feller AC, et al. Report of the Workshop on Nasal and Related Extranodal Angiocentric T/Natural Killer Cell Lymphomas. Definitions, differential diagnosis, and epidemiology. Am J Surg Pathol. 1996;20:103–11.
Ishii H, Takahara M, Nagato T, Kis LL, Nagy N, Kishibe K, et al. Monocytes enhance cell proliferation and LMP1 expression of nasal natural killer/T-cell lymphoma cells by cell contact-dependent interaction through membrane-bound IL-15. Int J Cancer. 2012;130:48–58.
Harabuchi Y, Yamanaka N, Kataura A, Imai S, Kinoshita T, Mizuno F, et al. Epstein-Barr virus in nasal T-cell lymphomas in patients with lethal midline granuloma. Lancet. 1990;335:128–30.
Minarovits J, Hu LF, Imai S, Harabuchi Y, Kataura A, Minarovits Kormuta S, et al. Clonality, expression and methylation patterns of the Epstein-Barr virus genomes in lethal midline granulomas classified as peripheral angiocentric T cell lymphomas. J Gen Virol. 1994;75:77–84.
Chiang AK, Tao Q, Srivastava G, Ho FC. Nasal NK- and T-cell lymphomas share the same type of Epstein-Barr virus latency as nasopharyngeal carcinoma and Hodgkin's disease. Int J Cancer. 1996;68:285–90.
Takahara M, Kishibe K, Bandoh N, Nonaka S, Harabuchi Y. P53, N- and K-Ras, and beta-catenin gene mutations and prognostic factors in nasal NK/T-cell lymphoma from Hokkaido, Japan. Hum Pathol. 2004;35:86–95.
Kaye KM, Izumi KM, Kieff E. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc Natl Acad Sci U S A. 1993;90:9150–4.
Wang D, Liebowitz D, Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985;43:831–40.
Nagato T, Kobayashi H, Kishibe K, Takahara M, Ogino T, Ishii H, et al. Expression of interleukin-9 in nasal natural killer/T-cell lymphoma cell lines and patients. Clin Cancer Res. 2005;11:8250–7.
Takahara M, Kis LL, Nagy N, Liu A, Harabuchi Y, Klein G, et al. Concomitant increase of LMP1 and CD25 (IL-2-receptor alpha) expression induced by IL-10 in the EBV-positive NK lines SNK6 and KAI3. Int J Cancer. 2006;119:2775–83.
Moriai S, Takahara M, Ogino T, Nagato T, Kishibe K, Ishii H, et al. Production of interferon-{gamma}-inducible protein-10 and its role as an autocrine invasion factor in nasal natural killer/T-cell lymphoma cells. Clin Cancer Res. 2009;15:6771–9.
Takahara M, Nagato T, Komabayashi Y, Yoshino K, Ueda S, Kishibe K, et al. Soluble ICAM-1 secretion and its functional role as an autocrine growth factor in nasal NK/T cell lymphoma cells. Exp Hematol. 2013;41:711–8.
Kumai T, Nagato T, Kobayashi H, Komabayashi Y, Ueda S, Kishibe K, et al. CCL17 and CCL22/CCR4 signaling is a strong candidate for novel targeted therapy against nasal natural killer/T-cell lymphoma. Cancer Immunol Immunother. 2015;64:697–705.
Yoshino K, Kishibe K, Nagato T, Ueda S, Komabayashi Y, Takahara M, et al. Expression of CD70 in nasal natural killer/T cell lymphoma cell lines and patients; its role for cell proliferation through binding to soluble CD27. Br J Haematol. 2013;160:331–42.
Komabayashi Y, Kishibe K, Nagato T, Ueda S, Takahara M, Harabuchi Y. Downregulation of miR-15a due to LMP1 promotes cell proliferation and predicts poor prognosis in nasal NK/T-cell lymphoma. Am J Hematol. 2014;89:25–33.
Nurse P. A long twentieth century of the cell cycle and beyond. Cell. 2000;100:71–78.
Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–66.
Altieri DC. The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr Opin Cell Biol. 2006;18:609–15.
O'Connor DS, Grossman D, Plescia J, Li F, Zhang H, Villa A, et al. Regulation of apoptosis at cell division by p34cdc2 phosphorylation of survivin. Proc Natl Acad Sci U S A. 2000;97:13103–7.
Goga A, Yang D, Tward AD, Morgan DO, Bishop JM. Inhibition of CDK1 as a potential therapy for tumors over-expressing MYC. Nat Med. 2007;13:820–7.
Bernasconi M, Ueda S, Krukowski P, Bornhauser BC, Ladell K, Dorner M, et al. Early gene expression changes by Epstein-Barr virus infection of B-cells indicate CDKs and survivin as therapeutic targets for post-transplant lymphoproliferative diseases. Int J Cancer. 2013;133:2341–50.
Ng SB, Selvarajan V, Huang G, Zhou J, Feldman AL, Law M, et al. Activated oncogenic pathways and therapeutic targets in extranodal nasal-type NK/T cell lymphoma revealed by gene expression profiling. J Pathol. 2011;223:496–510.
Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. TheWorld Health Organization classification of neoplasms of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting--Airlie House, Virginia, November, 1997. Hematol J. 2000;1:53–66.
Nagata H, Konno A, Kimura N, Zhang Y, Kimura M, Demachi A, et al. Characterization of novel natural killer (NK)-cell and gammadelta T-cell lines established from primary lesions of nasal T/NK-cell lymphomas associated with the Epstein-Barr virus. Blood. 2001;97:708–13.
Ladell K, Dorner M, Zauner L, Berger C, Zucol F, Bernasconi M, et al. Immune activation suppresses initiation of lytic Epstein-Barr virus infection. Cell Microbiol. 2007;9:2055–69.
Ueda S, Uchiyama S, Azzi T, Gysin C, Berger C, Bernasconi M, et al. Oropharyngeal group A streptococcal colonization disrupts latent Epstein-Barr virus infection. J Infect Dis. 2014;209:255–64.
Kanemitsu N, Isobe Y, Masuda A, Momose S, Higashi M, Tamaru J, et al. Expression of Epstein-Barr virus-encoded proteins in extranodal NK/T-cell Lymphoma, nasal type (ENKL): differences in biologic and clinical behaviors of LMP1-positive and -negative ENKL. Clin Cancer Res. 2012;18:2164–72.
Chen X, Zhang FH, Chen QE, Wang YY, Wang YL, He JC, et al. The clinical significance of CDK1 expression in oral squamous cell carcinoma. Med Oral Patol Oral Cir Bucal. 2015;20:e7–12.
Cho HJ, Kim HR, Park YS, Kim YH, Kim DK, Park SI. Prognostic value of survivin expression in stage III non-small cell lung cancer patients treated with platinum-based therapy. Surg Oncol. 2015;24:329–34.
Dong H, Qian D, Wang Y, Meng L, Chen D, Ji X, et al. Survivin expression and serum levels in pancreatic cancer. World J Surg Oncol. 2015;13:189.
Nagato T, Ohkuri T, Ohara K, Hirata Y, Kishibe K, Komabayashi Y, et al. Programmed death-ligand 1 and its soluble form are highly expressed in nasal natural killer/T-cell lymphoma: a potential rationale for immunotherapy. Cancer Immunol Immunother. 2017;66:877–90.
Chang CC, Heller JD, Kuo J, Huang RC. Tetra-O-methyl nordihydroguaiaretic acid induces growth arrest and cellular apoptosis by inhibiting Cdc2 and survivin expression. Proc Natl Acad Sci U S A. 2004;101:13239–44.
Suzuki M, Takeda T, Nakagawa H, Iwata S, Watanabe T, Siddiquey MN, et al. The heat shock protein 90 inhibitor BIIB021 suppresses the growth of T and natural killer cell lymphomas. Front Microbiol. 2015;6:280.
Sun L, Zhao Y, Shi H, Ma C, Wei L. LMP-1 induces survivin expression to inhibit cell apoptosis through the NF-kappaB and PI3K/Akt signaling pathways in nasal NK/T-cell lymphoma. Oncol Rep. 2015;33:2253–60.
Guo L, Tang M, Yang L, Xiao L, Bode AM, Li L, et al. Epstein-Barr virus oncoprotein LMP1 mediates survivin upregulation by p53 contributing to G1/S cell cycle progression in nasopharyngeal carcinoma. Int J Mol Med. 2012;29:574–80.
Ai MD, Li LL, Zhao XR, Wu Y, Gong JP, Cao Y. Regulation of survivin and CDK4 by Epstein-Barr virus encoded latent membrane protein 1 in nasopharyngeal carcinoma cell lines. Cell Res. 2005;15:777–84.
Endo K, Shackelford J, Aga M, Yoshizaki T, Pagano JS. Upregulation of special AT-rich-binding protein 1 by Epstein-Barr virus latent membrane protein 1 in human nasopharyngeal cells and nasopharyngeal cancer. J Gen Virol. 2013;94:507–13.
Diamantopoulos PT, Polonyfi K, Sofotasiou M, Mantzourani M, Galanopoulos A, Spanakis N, et al. Survivin messenger RNA levels in Epstein-Barr virus-positive patients with leukemic low-grade B-cell lymphomas expressing the latent membrane protein 1: evidence of apoptotic function? Clin Lymphoma Myeloma Leuk. 2014;14:56–60.
Chiu CY, Kuo KK, Kuo TL, Lee KT, Cheng KH. The activation of MEK/ERK signaling pathway by bone morphogenetic protein 4 to increase hepatocellular carcinoma cell proliferation and migration. Mol Cancer Res. 2012;10:415–27.
Roberts ML, Cooper NR. Activation of a ras-MAPK-dependent pathway by Epstein-Barr virus latent membrane protein 1 is essential for cellular transformation. Virology. 1998;240:93–99.
Heller JD, Kuo J, Wu TC, Kast WM, Huang RC. Tetra-O-methyl nordihydroguaiaretic acid induces G2 arrest in mammalian cells and exhibits tumoricidal activity in vivo. Cancer Res. 2001;61:5499–504.
Sewell IA, Ellis H. A trial of mithramycin in the treatment of advanced malignant disease. Br J Cancer. 1966;20:256–63.
Baum M. A clinical trial of mithramycin in the treatment of advanced malignant disease. Br J Cancer. 1968;22:176–83.
Ream NW, Perlia CP, Wolter J, Taylor SG 3rd. Mithramycin therapy in disseminated germinal testicular cancer. JAMA. 1968;204:1030–6.
Cheson BD, Bartlett NL, Vose JM, Lopez-Hernandez A, Seiz AL, Keating AT, et al. A phase II study of the survivin suppressant YM155 in patients with refractory diffuse large B-cell lymphoma. Cancer. 2012;118:3128–34.
Grossman SA, Ye X, Peereboom D, Rosenfeld MR, Mikkelsen T, Supko JG, et al. Phase I study of terameprocol in patients with recurrent high-grade glioma. Neuro Oncol. 2012;14:511–7.
Lanasa MC, Andritsos L, Brown JR, Gabrilove J, Caligaris-Cappio F, Ghia P, et al. Final results of EFC6663: a multicenter, international, phase 2 study of alvocidib for patients with fludarabine-refractory chronic lymphocytic leukemia. Leuk Res. 2015;39:495–500.
Tibes R, McDonagh KT, Lekakis L, Bogenberger JM, Kim S, Frazer N, et al. Phase I study of the novel Cdc2/CDK1 and AKT inhibitor terameprocol in patients with advanced leukemias. Invest New Drugs. 2015;33:389–96.
Zeidner JF, Foster MC, Blackford AL, Litzow MR, Morris LE, Strickland SA, et al. Randomized multicenter phase II study of flavopiridol (alvocidib), cytarabine, and mitoxantrone (FLAM) versus cytarabine/daunorubicin (7+3) in newly diagnosed acute myeloid leukemia. Haematologica. 2015;100:1172–9.
Yamaguchi M, Suzuki R, Oguchi M, Asano N, Amaki J, Akiba T, et al. Treatments and Outcomes of Patients With Extranodal Natural Killer/T-Cell Lymphoma Diagnosed Between 2000 and 2013: A Cooperative Study in Japan. J Clin Oncol. 2017;35:32–39.
Takahara M, Nagato T, Kishibe K, Ueda S, Komabayashi Y, Yamashina M, et al. Novel treatment for early-stage nasal natural killer/T-cell lymphoma: intra-maxillary arterial infusion chemotherapy with concomitant radiotherapy. Hematol Oncol. 2017;35:158–62.
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI [Grant Numbers 15K20172/18K09310 (TN), 15K20174 (SU), 16K15244 (HK), and 15H04986/18H02948 (YH)]. The authors thank Dr. Norio Shimizu (Tokyo Medical and Dental University) for generously providing cell lines, Mr. Toshiyuki Hayakawa (Animal Laboratory for Medical Research, Center for Advanced Research and Education, Asahikawa Medical University) for devotedly maintaining mice, and Ms. Rie Matsumoto (Department of Pathology, Asahikawa Medical University) and Ms. Keiko Nishikura (Department of Dermatology, Asahikawa Medical University) for technical assistance.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Nagato, T., Ueda, S., Takahara, M. et al. Cyclin-dependent kinase 1 and survivin as potential therapeutic targets against nasal natural killer/T-cell lymphoma. Lab Invest 99, 612–624 (2019). https://doi.org/10.1038/s41374-018-0182-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41374-018-0182-9
This article is cited by
-
Bioinformatics analysis of the pathogenic link between Epstein-Barr virus infection, systemic lupus erythematosus and diffuse large B cell lymphoma
Scientific Reports (2023)
-
Expression of soluble CD27 in extranodal natural killer/T-cell lymphoma, nasal type: potential as a biomarker for diagnosis and CD27/CD70-targeted therapy
Cancer Immunology, Immunotherapy (2023)