Abstract
Objective
To determine the proportion of infant deaths occurring in the setting of a confirmed genetic disorder.
Study design
A retrospective analysis of the electronic medical records of infants born from 1 January, 2011 to 1 June, 2017, who died prior to 1 year of age.
Results
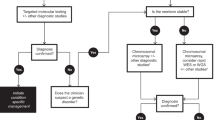
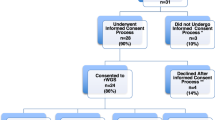
Five hundred and seventy three deceased infants were identified. One hundred and seventeen were confirmed to have a molecular or cytogenetic diagnosis in a clinical diagnostic laboratory and an additional seven were diagnosed by research testing for a total of 124/573 (22%) diagnosed infants. A total of 67/124 (54%) had chromosomal disorders and 58/124 (47%) had single gene disorders (one infant had both). The proportion of diagnoses made by sequencing technologies, such as exome sequencing, increased over the years.
Conclusions
The prevalence of confirmed genetic disorders within our cohort of infant deaths is higher than that previously reported. Increased efforts are needed to further understand the mortality burden of genetic disorders in infancy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Simpson CD, Ye XY, Hellmann J, Tomlinson C. Trends in cause-specific mortality at a Canadian outborn NICU. Pediatrics. 2010;126:1538.
Jacob J, Kamitsuka M, Clark RH, Kelleher AS, Spitzer AR. Etiologies of NICU deaths. Pediatrics. 2015;135:e59–65.
Meng M, Zhang YP. Impact of inborn errors of metabolism on admission in a neonatal intensive care unit: a 4-year report. J Pediatr Endocrinol Metab. 2013;26:689–93.
Wojcik MH, Schwartz TS, Yamin I, Edward HL, Genetti CA, Towne MC, et al. Genetic disorders and mortality in infancy and early childhood: delayed diagnoses and missed opportunities. Genet Med. 2018;20:1396–404.
Hudome SM, Kirby RS, Senner JW, Cunniff C. Contribution of genetic disorders to neonatal mortality in a regional intensive care setting. Am J Perinatol. 1994;11:100–3.
Weiner J, Sharma J, Lantos J, Kilbride H. How infants die in the neonatal intensive care unit: trends from 1999 through 2008. Arch Pediatr Adolesc Med. 2011;165:630–4.
Nelson K, Holmes LB. Malformations due to presumed spontaneous mutations in newborn infants. N Engl J Med. 1989;320:19–23.
Mathews TJ, Driscoll AK. Trends in infant mortality in the United States, 2005–2014. NCHS data brief, no 279. Hyattsville, MD: National Center for Health Statistics; 2017.
Murphy SL, Mathews TJ, Martin JA, Minkovitz CS, Strobino DM. Annual summary of vital statistics: 2013–2014. Pediatrics. 2017;139:3239.
Sankaran K, Chien LY, Walker R, Seshia M, Ohlsson A. Canadian Neonatal Network Variations in mortality rates among Canadian neonatal intensive care units. CMAJ. 2002;166:173–8.
Wen SW, Liu S, Joseph KS, Rouleau J, Allen A. Patterns of infant mortality caused by major congenital anomalies. Teratology. 2000;61:342–6.
Costa S, Rodrigues M, Centeno MJ, Martins A, Vilan A, Brandao O, et al. Diagnosis and cause of death in a neonatal intensive care unit-how important is autopsy? J Matern Fetal Neonatal Med. 2011;24:760–3.
Michel MC, Colaizy TT, Klein JM, Segar JL, Bell EF. Causes and circumstances of death in a neonatal unit over 20 years. Pediatr Res. 2018;83:829–33.
Seske LM, Muglia LJ, Hall ES, Bove KE, Greenberg JM. Infant mortality, cause of death, and vital records reporting in Ohio, United States. Matern Child Health J. 2017;21:727–33.
Meng L, Pammi M, Saronwala A, Magoulas P, Ghazi AR, Vetrini F, et al. Use of exome sequencing for infants in intensive care units: ascertainment of severe single-gene disorders and effect on medical management. JAMA Pediatr. 2017;171:e173438.
Saunders CJ, Miller NA, Soden SE, Dinwiddie DL, Noll A, Alnadi NA, et al. Rapid whole-genome sequencing for genetic disease diagnosis in neonatal intensive care units. Sci Transl Med. 2012;4:154ra135.
Smith LD, Willig LK, Kingsmore SF. Whole-exomesequencing and whole-genome sequencing in critically ill neonates suspected to have single-gene disorders. Cold Spring Harb Perspect Med. 2015;6:a023168.
Willig LK, Petrikin JE, Smith LD, Saunders CJ, Thiffault I, Miller NA, et al. Whole-genome sequencing for identification of Mendelian disorders in critically ill infants: a retrospective analysis of diagnostic and clinical findings. Lancet Respir Med. 2015;3:377–87.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81.
Centers for Disease Control and Prevention. 1.4 Congenital anomalies—definitions. https://www.cdc.gov/ncbddd/birthdefects/surveillancemanual/chapters/chapter-1/chapter1-4.html. Accessed 4 Oct 2018.
Joshi M, Anselm I, Shi J, Bale TA, Towne M, Schmitz-Abe K, et al. Mutations in the substrate binding glycine-rich loop of the mitochondrial processing peptidase-alpha protein (PMPCA) cause a severe mitochondrial disease. Cold Spring Harb Mol Case Stud. 2016;2:a000786.
Holm IA, Agrawal PB, Ceyhan-Birsoy O, Christensen KD, Fayer S, Frankel LA, et al. The BabySeq project: implementing genomic sequencing in newborns. BMC Pediatr. 2018;18:225.
Wojcik MH, Okada K, Prabhu SP, Nowakowski DW, Ramsey K, Balak C, et al. De novo variant in KIF26B is associated with pontocerebellar hypoplasia with infantile spinal muscular atrophy. Am J Med Genet A. 2018;176:2623–9.
Portnoï MF, Lebas F, Gruchy N, Ardalan A, Biran-Mucignat V, Malan V, et al. 22q11.2 duplication syndrome: two new familial cases with some overlapping features with DiGeorge/velocardiofacial syndromes. Am J Med Genet A. 2005;137:47–51.
Johnston AM, Niemela J, Rosenzweig SD, Fried AJ, Delmonte OM, Fleisher TA, et al. A novel mutation in IKBKG/NEMO leads to ectodermal dysplasia with severe immunodeficiency (EDA-ID). J Clin Immunol. 2016;36:541–3.
van Diemen CC, Kerstjens-Frederikse WS, Bergman KA, de Koning TJ, Sikkema-Raddatz B, van der Velde JK, et al. Rapid targeted genomics in critically ill newborns. Pediatrics. 2017;140:2854.
Stark Z, Tan TY, Chong B, Brett GR, Yap P, Walsh M, et al. A prospective evaluation of whole-exome sequencing as a first-tier molecular test in infants with suspected monogenic disorders. Genet Med. 2016;18:1090–6.
Petrikin JE, Willig LK, Smith LD, Kingsmore SF. Rapid whole genome sequencing and precision neonatology. Semin Perinatol. 2015;39:623–31.
Trowbridge A, Walter JK, McConathey E, Morrison W, Feudtner C. Modes of death within a children’s hospital. Pediatrics. 2018;142:e20174182.
McPherson E, Nestoridi E, Heinke D, Roberts DJ, Fretts R, Yazdy MM, et al. Alternatives to autopsy for fetal and early neonatal (perinatal) deaths: insights from the Wisconsin stillbirth service program. Birth Defects Res. 2017;109:1430–41.
Auger N, Bilodeau-Bertrand M, Poissant J, Shah PS. Decreasing use of autopsy for stillbirths and infant deaths: missed opportunity. J Perinatol. 2018;38:1414–9.
Wojcik MH, Brodsky D, Stewart JE, Picker J. Peri-mortem evaluation of infants who die without a diagnosis: focus on advances in genomic technology. J Perinatol. 2018;38:1125–34.
Flex E, Niceta M, Cecchetti S, Thiffault I, Au MG, Capuano A, et al. Biallelic Mutations in TBCD, encoding the tubulin folding cofactor D, perturb microtubule dynamics and cause early-onset encephalopathy. Am J Hum Genet. 2016;99:962–73.
Miyake N, Fukai R, Ohba C, Chihara T, Miura M, Shimizu H, et al. Biallelic TBCD mutations cause early-onset neurodegenerative encephalopathy. Am J Hum Genet. 2016;99:950–61.
Butcher DT, Cytrynbaum C, Turinsky AL, Siu MT, Inbar-Feigenberg M, Mendoza-Londono R, et al. CHARGE and Kabuki syndromes: gene-specific DNA methylation signatures identify epigenetic mechanisms linking these clinically overlapping conditions. Am J Hum Genet. 2017;100:773–88.
Khan SQ, Berrington de Gonzalez A, Best AF, Chen Y, Haozous EA, Rodriquez EJ, et al. Infant and youth mortality trends by race/ethnicity and cause of death in the United States. JAMA Pediatr. 2018;172:e183317.
Acknowledgements
We would like to thank the patients and families whom we care for at our hospital who continue to teach and enrich us all. MHW is supported by NIH T32 GM007748. PBA is supported by NICHD/NHGRI/NIH U19HD077671. CSG was supported by a Harvard University Milton Fund award. TWY is supported by NIH/NIMH R01MH113761 and NICHD/NHGRI/NIH U19HD077671. KET received research support from the Summer Student Research Program of the Division of Newborn Medicine at Boston Children’s Hospital and Harvard Medical School and from The Brody School of Medicine at East Carolina University. The Broad Center for Mendelian Genomics (UM1 HG008900) is funded by the National Human Genome Research Institute with supplemental funding provided by the National Heart, Lung, and Blood Institute under the Trans-Omics for Precision Medicine (TOPMed) program and the National Eye Institute. We appreciate the assistance of Anthony Capraro, MD (Department of Emergency Medicine, Boston Children’s Hospital) in identifying our cohort of deceased infants.
Funding
Supported by the National Institutes of Health (NIH T32 GM007748 [to MHW], and NICHD/NHGRI/NIH U19HD077671 [to PBA], NIH/NIMH R01MH113761 and NICHD/NHGRI/NIH U19HD077671 [to TWY]). Additional support provided by Harvard University (to CSG) and Boston Children’s Hospital/Harvard Medical School and from The Brody School of Medicine at East Carolina University (to KET). The Broad Center for Mendelian Genomics (UM1 HG008900) is funded by the National Human Genome Research Institute with supplemental funding provided by the National Heart, Lung, and Blood Institute under the Trans-Omics for Precision Medicine (TOPMed) program and the National Eye Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest relevant to this article to disclose (TEM is currently an employee of Quest Diagnostics who performed the work pertaining to this article while at the Broad Institute of MIT and Harvard).
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wojcik, M.H., Schwartz, T.S., Thiele, K.E. et al. Infant mortality: the contribution of genetic disorders. J Perinatol 39, 1611–1619 (2019). https://doi.org/10.1038/s41372-019-0451-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-019-0451-5
This article is cited by
-
Role of genomic medicine and implementing equitable access for critically ill infants in neonatal intensive care units
Journal of Perinatology (2023)
-
A model to implement genomic medicine in the neonatal intensive care unit
Journal of Perinatology (2023)
-
Genomic newborn screening for rare diseases
Nature Reviews Genetics (2023)
-
Rare diseases, common barriers: disparities in pediatric clinical genetics outcomes
Pediatric Research (2023)
-
Knockout mouse models as a resource for the study of rare diseases
Mammalian Genome (2023)