Abstract
Background
Testosterone treatment is generally not recommended in men with obesity induced low serum testosterone. However, distinguishing this condition from overt testosterone deficiency in men with obesity where treatment should be initiated is a diagnostic challenge and tools to differentiate these conditions are scarce but could be of important clinical relevance.
Objectives
To investigate the association between body composition and dynamic responses of the pituitary-testis axis in men.
Methods
Single-center cross-sectional study including 112 healthy men. Participants went through a full biochemical assessment of the pituitary-testis axis, and dynamic stimulatory tests of luteinizing hormone (LH) secretion (gonadotropin-releasing hormone (GnRH)-test) and testosterone secretion (choriogonadotropin (hCG)-test). A subset (N = 78) further had a DXA-scan performed.
Results
A higher body mass index (BMI) was associated with lower basal serum LH (BU = −0.44, 95% CI: −0.88–−0.01, p = 0.04). The GnRH-stimulated LH increase was not significantly associated with BMI (BU = −0.10, 95% CI: −0.72–0.51, p = 0.74). Furthermore, a high BMI was associated with low basal testosterone (BU −0.02, 95% CI: −0.03–−0.02, p < 0.001), and free testosterone (BU −15.0, 95% CI: −19.9–−10.0, p < 0.001) and men with overweight and obesity had significantly lower testosterone (9%, p = 0.003 and 24%, p < 0.001) and free testosterone (25%, p = 0.006 and 50%, p < 0.001) concentrations compared to men with normal weight. The HCG-stimulated testosterone increase was significantly less dependent on BMI compared to the influence of BMI on basal testosterone concentrations (p = 0.04 for the interaction).
Conclusions
Dynamic sex hormone responses following pituitary-testis axis stimulation were less dependent on BMI, compared to the influence of BMI on basal hormone concentrations and could potentially assist clinical decision making in patients with obesity suspected of testosterone deficiency.
Similar content being viewed by others
Introduction
The prevalence of obesity is drastically increasing, reaching pandemic heights with more than 1 billion people worldwide living with obesity (https://www.who.int/news/item/04-03-2022-world-obesity-day-2022-accelerating-action-to-stop-obesity). Several studies suggest a strong association between serum testosterone (T) concentrations and excess body weight in men [1,2,3]. However, the interplay between body composition and functioning of the hypothalamic-pituitary-gonadal (HPG) axis is complex and not fully understood and several mechanisms are thought to contribute to obesity-induced inhibition of the HPG axis. Thus, obesity, particularly central obesity and steatosis, inhibits production of sex hormone binding globulin (SHBG) [4, 5] contributing to lower circulating total T, through a lower T binding capacity, with only little affection of the free testosterone (FT) concentration [5]. Furthermore, increased adipose tissue-related aromatization of T to estradiol (E2) is believed to contribute to lower serum T and higher E2, to a varying extent, potentially increasing negative feedback at the hypothalamo-pituitary level, whereby T production is inhibited [6, 7]. Lastly, obesity associated chronic inflammation and adipokine signaling has been suggested to impair gonadotropin secretion, Leydig cell function and T production [8].
T treatment is generally not recommended in cases of obesity-induced low serum T [9]. Thus, distinguishing this condition from overt T deficiency, where treatment should be initiated, is of clinical importance. However, this task is complicated and tools to assist clinical decision making are requested.
Dynamic testing of the HPG axis can be used to support the diagnostic process of T deficiency [10, 11]. The maximum gonadotropin production from the pituitary gland can be assessed by the gonadotropin-releasing hormone (GnRH) test, whereas the maximum capacity of the Leydig cells’ T production can be assessed by the human chorionic gonadotropin (hCG) test. However, it is largely unknown how body weight affects the HPG response upon stimulation. Thus, this study was conducted to investigate the association between body mass index (BMI) and dynamic responses of the HPG axis upon stimulation in a healthy cohort of men.
Methods
Study population
The study population was healthy men from the general Danish population examined between year 2012 and 2014. All men were participating in an ongoing study of testicular function, where the men underwent a general health examination, completed questionnaires concerning general health and lifestyle factors and a subset delivered a semen sample [11, 12]. The included men were in addition to standard testing, invited to take part in dynamic tests of the HPG axis. Participants who either had a hCG test, a GnRH test, or both performed within the test period were included in this study. A subset of the participants also went through a full-body DXA-scan. Exclusion criteria were 1) chronic diseases, 2) medical history of testicular surgery or trauma, 3) current use of anabolic steroids. All examinations, tests and laboratory assessments were performed at Department of Growth and Reproduction, Rigshospitalet, Copenhagen, Denmark. The study was approved by the ‘Ethical committee of the Capital region’ (permit number H-KF-289428) and performed according to the Declaration of Helsinki. Individual consent was obtained verbally and in writing from all participants.
Reproductive hormone analyses
Serum concentrations of LH and T were determined using a time-resolved fluoroimmunoassay (Delfia, Wallac, Turku, Finland). E2 concentrations were determined using radioimmunoassay (Biotech-IGG, Pantex). SHBG was determined by time-resolved chemiluminescent immunoassay (Access, Beckman Coulter). Inter- and intraassay coefficients of variation (CVs) for measurements of the hormones for LH were 2 and 3%, SHBG 5 and 4%, T 10 and 6%, and for E2 15 and 8%. FT was calculated using the Vermeulen formula with a fixed albumin (43.8 g/L) [13]. Total testosterone was considered as low with serum concentrations below 10 nmol/L and free testosterone was considered low at serum concentrations below 200 pmol/L based on laboratory specific reference ranges. All analyses were validated and accredited by the Danish Accreditation Fund (DANAK, www.danak.dk).
HPG stimulation tests
Reproductive hormone testing was performed as described previously [11]. GnRH and hCG tests were initiated between 08:00 and 12:00 h. Initially, a common baseline blood sample for both the GnRH and hCG test was drawn for measurement of LH and T. LH and T increases were calculated as the difference between basal and stimulated serum concentrations as we previously showed that these measurements are a valuable diagnostic tool in the evaluation of HPG disorders [11]. GnRH stimulation test: 100 μg GnRH (Relefact, Sanofi-Aventis, Frankfurt, Germany) was given intravenously, and a blood sample was collected after 30 min for LH measurement. HCG stimulation test: Was initiated immediately following the GnRH test. An injection of 5000 IU hCG (Pregnyl, Organon, Amsterdam, Netherlands) was given in the gluteal muscle. A follow-up blood sample was taken 72 h later, with an allowed variation of +/−1 h, for measurement of T.
Body composition
Fat and lean body masses were assessed through a DXA scan (Lunar Prodigy Advance; GE Medical Systems Lunar, Milwaukee, WI, USA). Software (Prodigy, enCORE 2004, version 8.8; GE Lunar Corp., Madison, WI, USA) was used to estimate regional and total fat and fat-free tissue masses. CVs were 2% for total body fat and regional body fat, measured on humans.
Statistical analyses
Continuous variables are displayed as medians with interquartile ranges (IQR) unless otherwise stated. Data were log10-transformed when not normally distributed. Histograms and Q–Q plots were used to assess normal distribution. Analyses of the association between body composition and basal and stimulated sex hormone concentrations were performed using a linear regression model. Models were checked for assumptions of the linear model, including normal distribution of the residuals, linearity, and homogeneity of variance. If a statistically significant association was observed, a best-fit curve was added to the figure including 95% confidence intervals. Interaction analyses were performed to investigate whether the association between BMI and basal serum hormone concentrations differed significantly from that of BMI and stimulated serum hormone concentrations. Participants were subdivided into three subgroups based on BMI: 1) men with normal weight (BMI < 25 kg/m2), 2) overweight (BMI 25–29.9 kg/m2), and 3) obesity (BMI ≥ 30 kg/m2). Basal and stimulated serum sex hormone concentrations were compared between subgroups using a one-way ANOVA. A Bonferroni post-hoc test was performed to assess between group differences. A two-way ANOVA was performed to estimate the effects of two independent categorical variables (high/low BMI: BMI < 25 kg/m2 versus BMI ≥ 25 kg/m2 and high/low FT: FT < 200 pmol/L versus FT ≥ 200 pmol/L) on dynamic test responses of the HPG axis. Single mediation analyses were conducted using the PROCES plug-in (v4.2). Statistical analyses were performed using IBM SPSS statistics version 28. A p-value < 0.05 was considered statistically significant.
Results
Subject characteristics
Study participants had a median age of 30.5 years (IQR: 19.0–35.5) and a BMI of 24.0 kg/m2 (range: 17.3–59.1 kg/m2). A BMI < 25 kg/m2 was registered in 65 men, 29 had a BMI between 25–29.9 kg/m2, and 18 had a BMI ≥ 30 kg/m2. Median T was 16.4 nmol/L (IQR: 11.9–21.3 nmol/L), FT was 369 pmol/L (IQR: 256–493 pmol/L) and LH 3.4 IU/L (IQR: 2.6–4.5 IU/L). Twenty-one men had a serum T considered low (T < 10 nmol/L) and 19 men had a FT considered low (FT < 200 pmol/L). All men were Danish citizens. Subject characteristics are shown in Table 1.
Association between BMI, and basal LH and GnRH-stimulated LH increase
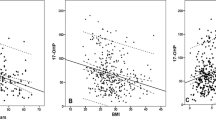
BMI explained 4% of the variation in basal LH (BU = −0.44, 95% CI: −0.88–−0.01, p = 0.04), where a higher BMI was associated with lower basal LH (Fig. 1A). A single-mediation analysis revealed that 14% (95% CI: 1–36%) of the negative relationship between BMI and LH was statistically mediated through serum E2. Basal LH concentrations did not differ significantly between BMI sub-groups (p = 0.16) (Fig. 1B). BMI was not significantly associated with the GnRH-stimulated LH increase (BU = −0.10, 95% CI: −0.72–0.51, p = 0.74) (Fig. 1C) and GnRH-stimulated LH concentrations did not differ between BMI sub-groups (p = 0.70) (Fig. 1D).
A Basal LH in relation to BMI in healthy men (n = 112). B Basal LH in men with normal weight (BMI < 25 kg/m2, n = 65), overweight (BMI 25–29.9 kg/m2, n = 29), and obesity ( ≥ 30 kg/m2, n = 18). C The GnRH-stimulated LH increase in relation to BMI in healthy men (n = 112). D The GnRH-stimulated LH increase in men with normal weight (BMI < 25 kg/m2, n = 65), overweight (BMI 25–29.9 kg/m2, n = 29), and obesity ( ≥ 30 kg/m2, n = 18). Every dot or arrow represents one person. Line represents a significant association including the 95% confidence interval.
Association between BMI, and basal T, and HCG-stimulated T increase
BMI explained 35% of the variation in basal serum T (BU −0.02, 95% CI: −0.03–−0.02, p < 0.001), where a higher BMI was associated with a lower basal serum T (Fig. 2A). Serum T was 9% (95% CI: 5–14%, p = 0.003) lower in men with overweight and 24% (95% CI: 19–30%, p < 0.001) lower in men with obesity compared to men with normal weight (Fig. 2B). Single-mediation analyses revealed that 17% (95% CI: 5–33%) of the negative relationship between BMI and testosterone was statistically mediated through serum SHBG. BMI explained 25% of the variation in FT (BU −15.0, 95% CI: −19.9–−10.0, p < 0.001), where a higher BMI was associated with a lower basal serum FT (Fig. 2C). Serum FT was 25% (95% CI: 16–34%, p = 0.006) lower in men with overweight and 50% (95% CI: 39–62%, p < 0.001) lower in men with obesity compared to men with normal weight (Fig. 2D). BMI explained 5% of the variation in the HCG-stimulated T increase (BU −0.36, 95% CI: −0.69–−0.03, p = 0.04, Fig. 2E). The relationship between BMI and HCG-stimulated testosterone was not statistically mediated through SHBG (20%, 95% CI: −12–71%). The negative association between BMI and basal T was significantly steeper compared to the negative association between BMI and HCG-stimulated T (p = 0.04 for the interaction). HCG-stimulated T was not significantly different in men with overweight (0%, 95% CI: −15–17%, p = 0.99) and obesity (−21%, 95% CI: −57–14%, p = 0.26) compared with men with normal weight (Fig. 2F).
A Basal T in relation to BMI in healthy men (n = 112). B Basal T in men with normal weight (BMI < 25 kg/m2, n = 65), overweight (BMI 25–29.9 kg/m2, n = 29), and obesity (≥30 kg/m2, n = 18). C Basal FT in relation to BMI in healthy men (n = 112). D Basal FT in men with normal weight (BMI < 25 kg/m2, n = 65), overweight (BMI 25–29.9 kg/m2, n = 29), and obesity (≥30 kg/m2, n = 18). E The HCG-stimulated T increase in relation to BMI in healthy men (n = 91). F The HCG-stimulated T increase in men with normal weight (BMI < 25 kg/m2, n = 55), overweight (BMI 25–29.9 kg/m2, n = 22), and obesity (≥30 kg/m2, n = 14). Every dot or arrow represents one person. Line represents a significant association including the 95% confidence interval. T testosterone. *Significantly different from normal weight, p < 0.05.
Dynamic sex hormone responses in men with low versus normal FT
The GnRH-stimulated LH increase in men with low basal FT did not differ significantly from men with normal basal FT (p = 0.41 for effect of FT), in either men with a normal BMI or men with a higher BMI (p = 0.96 for interaction) (Fig. 3A). Accordingly, the HCG-stimulated T increase in men with low basal FT did not differ significantly from men with normal basal FT (p = 0.21 for effect of FT). Interaction analyses could not be performed due to lack of data on men with normal weight and low FT (Fig. 3B).
A The GnRH-stimulated LH increase in men with normal FT, BMI < 25 kg/m2: n = 62. Low FT, BMI < 25 kg/m2: n = 3. Normal FT, BMI ≥ 25 kg/m2: n = 31. Low FT, BMI ≥ 25 kg/m2: n = 16. B The HCG-stimulated T increase in men with normal FT, BMI < 25 kg/m2: n = 55. Low FT, BMI < 25 kg/m2: n = 0 (missing data). Normal FT, BMI ≥ 25 kg/m2: n = 28. Low FT, BMI ≥ 25 kg/m2: n = 8.
Association between fat distribution, and basal and stimulated LH and T
We used DXA-scans to investigate the association between regional adiposity and basal versus stimulated sex hormone responses. Trunk fat was significantly associated with basal LH (BU = −0.02, 95% CI: −0.03–0.00, p = 0.03), total T (BU = −0.02, 95% CI: −0.03–0.00, p = 0.02), and FT (BU = −15.0, 95% CI: −27.2– −2.7, p = 0.02), where a higher trunk fat mass was associated with lower basal serum LH, T, and FT. Trunk fat mass was not significantly associated with GnRH-stimulated LH (BU = −0.77, 95% CI: −1.69–0.14, p = 0.10) or HCG-stimulated T (BU = −0.45, 95% CI: −1.33–0.43, p = 0.31). Limb fat was not significantly associated with either basal or stimulated LH or T (Table 2).
Discussion
Distinguishing obesity-induced low concentrations of total T from overt T deficiency, where T treatment is indicated, can be a challenging task. Diagnostic tools to assist clinical decisions are therefore highly requested. We previously showed that dynamic testing of the HPG axis can be used as a tool to support the diagnostic process of T deficiency [11]. However, the effect of body composition on these tests have remained unknown.
In this study of 112 healthy men, we found that the dynamic sex hormone responses following HPG axis stimulation were less dependent on BMI, compared to the influence of BMI on basal serum sex hormone concentrations. Thus, a high BMI was associated with low basal LH, but the GnRH-stimulated LH increase was not significantly associated with body composition. Furthermore, the negative association between BMI and basal serum T was significantly steeper compared to the negative association between BMI and the HCG-stimulated T increase.
In accordance with previous studies, we detected that both basal total T and FT was closely associated with BMI [14,15,16,17]. Stratifying basal T concentrations by BMI class confirmed a stepwise decrease in basal T and FT with increasing BMI class, whereas the HCG-stimulated T increase was not significantly different in men with either overweight or obesity compared to men with normal weight. However, we did see a trend towards a lower HCG-stimulated T increase in a subgroup of the men with obesity, suggesting a considerable individual variation in the effects of adipose inhibition on the dynamic response of the pituitary-testis axis, within this group. Several studies indicate that the metabolic burden related to obesity varies substantially among individuals [18,19,20]. Thus, up to 25–50% of people with obesity are estimated to have a metabolically healthy phenotype, despite the higher body weight [21]. We speculate, that obesity with a higher metabolic burden (metabolically unhealthy obesity) could impact dynamic pituitary-gonadal responses to a greater extent, as a high trunk fat mass was associated with lower serum sex hormone concentrations whereas limb fat was not. This is in accordance with previous studies indicating a particularly significant role of visceral fat in the adipose inhibition of the HPG axis, beyond steroid hormone aromatization and regulation of SHBG [22,23,24,25]. We found, that serum E2 and SHBG only partly statistically mediated basal sex hormone concentrations and did not statistically mediate dynamic sex hormone responses, suggesting that additional mechanisms contribute to the crosstalk between adipose tissue and the HPG axis. In relation to this, obesity-related inflammation has been shown to inhibit HPG-signaling and blockade of central inflammatory pathways, in men with obesity and low serum T, increase T concentrations [26]. Several studies further suggest substantial adipokine signaling from the adipose tissue to the HPG axis through e.g., leptin and adiponectin [27,28,29].
The association between BMI and LH was generally modest compared to the association between BMI and testosterone. Previous studies suggest that severe obesity (BMI ≥ 40 kg/m2) is required to suppress pituitary gonadotropin secretion [30, 31]. In this study, only two men had a BMI ≥ 40 kg/m2 which prevent us from drawing conclusions in cases of class III obesity. However, one previous study of 10 men with class III obesity confirms substantial stimulatory HPG axis reserve in this group of men as well [32]. Nonetheless, we did find a significant association between a higher BMI and lower basal LH which was partly statistically mediated through higher serum E2. While studies indicate that serum E2 concentrations directly reflect the negative feedback exerted by estrogens on gonadotropin release, the effects of excess adiposity on serum E2 remains debated [33,34,35].
Obesity is associated with several endocrine abnormalities arising from changes in the hypothalamo-pituitary axis [36]. For example, obesity attenuates the stimulated growth hormone response [37], leads to ACTH hyperresponsiveness [38], and is associated with thyroid dysfunction [39]. Thus, it is no surprise that also the interpretation of the HPG axis functioning is complicated by obesity.
Whether T treatment is beneficial in men with obesity and low serum T concentrations is controversial [9, 40, 41]. Considering the limited number of studies showing beneficial effects, T therapy is currently not recommended in the prevention of metabolic disturbances associated with obesity and guidelines recommend that only patients with low FT and symptoms of T deficiency should be considered T deficient [3, 9].
This study was performed on healthy men with no symptoms of T deficiency. In a clinical setting, a patient might have T deficiency, which could impact the relationship between body composition and HPG axis functioning differently [42]. However, the most common challenge is to identify and sort out the man with obesity-induced low T, who does not need further T treatment. The findings of this study indicate that dynamic testing of the HPG axis could be a valuable tool to identify the otherwise healthy man with obesity-related low serum T, because stimulation of the HPG axis seems less sensitive to body composition. Thus, a normal response of the GnRH-test as well as the HCG-test in a man with overweight or obesity will suggest that his low serum T concentration is due to excess adiposity rather than overt T deficiency. Thus, our results are, to the best of our knowledge, the first to evaluate the potential impact of overweight and obesity on the dynamic sex hormone stimulation tests and the first to provide suggestions for a tool to differentiate between obesity-induced low serum T concentrations versus overt T deficiency in men with excess bodyweight.
Our study has some limitations. The study was based on observational data and, consequently, causal conclusions could not be drawn. Only 18 out of 112 men had a BMI ≥ 30 kg/m2, which affects the precision of the estimates in men with obesity. Furthermore, low FT in men with normal weight was rare and conclusions on dynamic test responses in this group could therefore not be drawn. Sex hormone measurements were performed using immunoassays. Whereas this only impacts accuracy of serum T measurements, within the normal range, to a small extent, serum concentrations of E2 measured using immunoassays are less accurate compared to mass spectrometry [43]. Furthermore, FT was calculated from Vermeulen’s formula, taking serum SHBG and albumin into account, and not measured from equilibrium dialysis, the gold standard. However, these circumstances often reflect the clinical reality, where advanced mass spectrometry and equilibrium dialysis equipment is not available. Excessive fat mass might inhibit hypothalamic secretion of GnRH through negative feedback from E2 [44]. We did not have data from clomiphene testing and therefore could not investigate the effects of bodyweight on dynamic sex hormone secretion at the hypothalamic level.
In conclusion, basal sex hormone concentrations are closely associated with BMI making the diagnostic evaluation of T deficiency complicated in patients with concomitant overweight and obesity. Dynamic sex hormone responses following HPG axis stimulation are less dependent on BMI and may therefore serve as a potentially valuable tool to assist clinical decision making in patients with overweight and obesity suspected of T deficiency.
Data availability
The datasets analyzed during the current study are not publicly available due to national data security legislation but are available from the corresponding author on reasonable request and with permission from the ethical committee of the capital region of Denmark.
References
Tajar A, Forti G, O’Neill TW, Lee DM, Silman AJ, Finn JD, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810–8.
Calderon B, Gomez-Martin JM, Vega-Pinero B, Martin-Hidalgo A, Galindo J, Luque-Ramirez M, et al. Prevalence of male secondary hypogonadism in moderate to severe obesity and its relationship with insulin resistance and excess body weight. Andrology. 2016;4:62–7.
Mangolim AS, Brito LAR, Nunes-Nogueira VDS. Effectiveness of testosterone replacement in men with obesity: a systematic review and meta-analysis. Eur J Endocrinol. 2021;186:123–35.
Selva DM, Hogeveen KN, Innis SM, Hammond GL. Monosaccharide-induced lipogenesis regulates the human hepatic sex hormone-binding globulin gene. J Clin Invest. 2007;117:3979–87.
Ruth KS, Day FR, Tyrrell J, Thompson DJ, Wood AR, Mahajan A, et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med. 2020;26:252–8.
Rao PM, Kelly DM, Jones TH. Testosterone and insulin resistance in the metabolic syndrome and T2DM in men. Nat Rev Endocrinol. 2013;9:479–93.
Nimrod A, Ryan KJ. Aromatization of androgens by human abdominal and breast fat tissue. J Clin Endocrinol Metab. 1975;40:367–72.
Tremellen K, McPhee N, Pearce K, Benson S, Schedlowski M, Engler H. Endotoxin-initiated inflammation reduces testosterone production in men of reproductive age. Am J Physiol Endocrinol Metab. 2018;314:E206–E13.
Corona G, Goulis DG, Huhtaniemi I, Zitzmann M, Toppari J, Forti G, et al. European Academy of Andrology (EAA) guidelines on investigation, treatment and monitoring of functional hypogonadism in males: Endorsing organization: European Society of Endocrinology. Andrology. 2020;8:970–87.
Cailleux-Bounacer A, Reznik Y, Cauliez B, Menard JF, Duparc C, Kuhn JM. Evaluation of endocrine testing of Leydig cell function using extractive and recombinant human chorionic gonadotropin and different doses of recombinant human LH in normal men. Eur J Endocrinol. 2008;159:171–8.
Bang AK, Nordkap L, Almstrup K, Priskorn L, Petersen JH, Rajpert-De Meyts E, et al. Dynamic GnRH and hCG testing: establishment of new diagnostic reference levels. Eur J Endocrinol. 2017;176:379–91.
Jorgensen N, Joensen UN, Jensen TK, Jensen MB, Almstrup K, Olesen IA, et al. Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men. BMJ Open. 2012;2:e000990.
Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–72.
Bann D, Wu FC, Keevil B, Lashen H, Adams J, Hardy R, et al. Changes in testosterone related to body composition in late midlife: Findings from the 1946 British birth cohort study. Obesity. 2015;23:1486–92.
Han S, Jeon YJ, Lee TY, Park GM, Park S, Kim SC. Testosterone is associated with abdominal body composition derived from computed tomography: a large cross sectional study. Sci Rep. 2022;12:22528.
Mouser JG, Loprinzi PD, Loenneke JP. The association between physiologic testosterone levels, lean mass, and fat mass in a nationally representative sample of men in the United States. Steroids. 2016;115:62–6.
Gates MA, Mekary RA, Chiu GR, Ding EL, Wittert GA, Araujo AB. Sex steroid hormone levels and body composition in men. J Clin Endocrinol Metab. 2013;98:2442–50.
Aguilar-Salinas CA, Garcia EG, Robles L, Riano D, Ruiz-Gomez DG, Garcia-Ulloa AC, et al. High adiponectin concentrations are associated with the metabolically healthy obese phenotype. J Clin Endocrinol Metab. 2008;93:4075–9.
Zheng R, Zhou D, Zhu Y. The long-term prognosis of cardiovascular disease and all-cause mortality for metabolically healthy obesity: a systematic review and meta-analysis. J Epidemiol Commun Health. 2016;70:1024–31.
Calori G, Lattuada G, Piemonti L, Garancini MP, Ragogna F, Villa M, et al. Prevalence, metabolic features, and prognosis of metabolically healthy obese Italian individuals: the Cremona Study. Diabetes Care. 2011;34:210–5.
Smith GI, Mittendorfer B, Klein S. Metabolically healthy obesity: facts and fantasies. J Clin Invest. 2019;129:3978–89.
Pitteloud N, Mootha VK, Dwyer AA, Hardin M, Lee H, Eriksson KF, et al. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care. 2005;28:1636–42.
Tsai EC, Boyko EJ, Leonetti DL, Fujimoto WY. Low serum testosterone level as a predictor of increased visceral fat in Japanese-American men. Int J Obes Relat Metab Disord. 2000;24:485–91.
Haffner SM, Valdez RA, Stern MP, Katz MS. Obesity, body fat distribution and sex hormones in men. Int J Obes Relat Metab Disord. 1993;17:643–9.
Kahn D, Macias E, Zarini S, Garfield A, Zemski Berry K, MacLean P, et al. Exploring Visceral and Subcutaneous Adipose Tissue Secretomes in Human Obesity: Implications for Metabolic Disease. Endocrinology. 2022;163:bqac140.
Ebrahimi F, Urwyler SA, Straumann S, Doerpfeld S, Bernasconi L, Neyer P, et al. IL-1 Antagonism in Men With Metabolic Syndrome and Low Testosterone: A Randomized Clinical Trial. J Clin Endocrinol Metab. 2018;103:3466–76.
Elsaied MA, Masallat D, Abdel-Hamid IA. Correlation of Adiponectin With Testosterone in Patients With and Without Type 2 Diabetes and Erectile Dysfunction. Am J Mens Health. 2019;13:1557988318807049.
Caminos JE, Nogueiras R, Gaytan F, Pineda R, Gonzalez CR, Barreiro ML, et al. Novel expression and direct effects of adiponectin in the rat testis. Endocrinology. 2008;149:3390–402.
Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18:298–303.
Giagulli VA, Kaufman JM, Vermeulen A. Pathogenesis of the decreased androgen levels in obese men. J Clin Endocrinol Metab. 1994;79:997–1000.
Vermeulen A, Kaufman JM, Deslypere JP, Thomas G. Attenuated luteinizing hormone (LH) pulse amplitude but normal LH pulse frequency, and its relation to plasma androgens in hypogonadism of obese men. J Clin Endocrinol Metab. 1993;76:1140–6.
Glass AR, Swerdloff RS, Bray GA, Dahms WT, Atkinson RL. Low serum testosterone and sex-hormone-binding-globulin in massively obese men. J Clin Endocrinol Metab. 1977;45:1211–9.
Raven G, de Jong FH, Kaufman JM, de Ronde W. In men, peripheral estradiol levels directly reflect the action of estrogens at the hypothalamo-pituitary level to inhibit gonadotropin secretion. J Clin Endocrinol Metab. 2006;91:3324–8.
Ghanim H, Dhindsa S, Abuaysheh S, Batra M, Kuhadiya ND, Makdissi A, et al. Diminished androgen and estrogen receptors and aromatase levels in hypogonadal diabetic men: reversal with testosterone. Eur J Endocrinol. 2018;178:277–83.
Corona G, Rastrelli G, Monami M, Saad F, Luconi M, Lucchese M, et al. Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: a systematic review and meta-analysis. Eur J Endocrinol. 2013;168:829–43.
van Hulsteijn LT, Pasquali R, Casanueva F, Haluzik M, Ledoux S, Monteiro MP, et al. Prevalence of endocrine disorders in obese patients: systematic review and meta-analysis. Eur J Endocrinol. 2020;182:11–21.
Dichtel LE, Yuen KC, Bredella MA, Gerweck AV, Russell BM, Riccio AD, et al. Overweight/Obese adults with pituitary disorders require lower peak growth hormone cutoff values on glucagon stimulation testing to avoid overdiagnosis of growth hormone deficiency. J Clin Endocrinol Metab. 2014;99:4712–9.
Pasquali R, Gagliardi L, Vicennati V, Gambineri A, Colitta D, Ceroni L, et al. ACTH and cortisol response to combined corticotropin releasing hormone-arginine vasopressin stimulation in obese males and its relationship to body weight, fat distribution and parameters of the metabolic syndrome. Int J Obes Relat Metab Disord. 1999;23:419–24.
Biondi B. Thyroid and obesity: an intriguing relationship. J Clin Endocrinol Metab. 2010;95:3614–7.
Jones TH, Arver S, Behre HM, Buvat J, Meuleman E, Moncada I, et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study). Diabetes Care. 2011;34:828–37.
Basaria S, Harman SM, Travison TG, Hodis H, Tsitouras P, Budoff M, et al. Effects of Testosterone Administration for 3 Years on Subclinical Atherosclerosis Progression in Older Men With Low or Low-Normal Testosterone Levels: A Randomized Clinical Trial. JAMA. 2015;314:570–81.
Grossmann M. Hypogonadism and male obesity: Focus on unresolved questions. Clin Endocrinol. 2018;89:11–21.
Huhtaniemi IT, Tajar A, Lee DM, O’Neill TW, Finn JD, Bartfai G, et al. Comparison of serum testosterone and estradiol measurements in 3174 European men using platform immunoassay and mass spectrometry; relevance for the diagnostics in aging men. Eur J Endocrinol. 2012;166:983–91.
Welt CK, Pagan YL, Smith PC, Rado KB, Hall JE. Control of follicle-stimulating hormone by estradiol and the inhibins: critical role of estradiol at the hypothalamus during the luteal-follicular transition. J Clin Endocrinol Metab. 2003;88:1766–71.
Acknowledgements
We appreciate the skilled and valuable work by the technicians in the hormone laboratory at the Department of Growth and Reproduction, Copenhagen University Hospital Rigshospitalet, Copenhagen, Denmark.
Funding
Open access funding provided by National Hospital.
Author information
Authors and Affiliations
Contributions
JA, AKB, LN, LP collected the data, JA, AKB, LN, LP, NJ analyzed the data, JA, NJ wrote the first draft of the paper, all authors contributed to writing and editing the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abildgaard, J., Bang, A.K., Nordkap, L. et al. The influence of body composition on the response to dynamic stimulation of the endocrine pituitary-testis axis. Int J Obes (2024). https://doi.org/10.1038/s41366-024-01518-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41366-024-01518-2