Abstract
Background
Physical activity improves insulin sensitivity in obesity. Hypoxia training is claimed to augment this effect. We tested the hypothesis that normobaric hypoxia training would improve insulin sensitivity in obese patients with metabolic syndrome.
Methods
In a randomized controlled trial, 23 obese men with metabolic syndrome who were not informed of the FiO2 conditions underwent a 6-week physical exercise intervention under ambient (n = 11; FiO2 21%) conditions or hypoxia (n = 12; FiO2 15%) using a normobaric hypoxic chamber. Three 60-min sessions of interval training were performed each week at 60% of individual V̇O2max. Assessment of myocellular insulin sensitivity by euglycemic hyperinsulinemic clamp was performed in 21 of these subjects before and after 6 weeks of training. Comprehensive phenotyping also included biopsies of subcutaneous adipose tissues.
Results
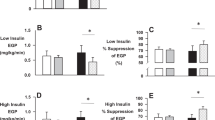
The intermittent moderate physical exercise protocol did not substantially change the myocellular insulin sensitivity within 6 weeks under normoxic conditions (ISIClamp: 0.035 (IQR 0.016–0.075) vs. 0.037 (IQR 0.026–0.056) mg* kg−1 *min−1/(mU* l−1); p = 0.767). In contrast, ISIClamp improved during hypoxia training (0.028 (IQR 0.018–0.035) vs. 0.038 (IQR 0.024–0.060) mg * kg−1 *min−1/(mU *l−1); p < 0.05). Between group comparison of ISIClamp change revealed a small difference between groups (Cohen's d = 0.26). Within the hypoxic group, improvement of ISIClamp during training was associated with individual increase of circulating vascular endothelial growth factor (VEGF) levels (r = 0.678, p = 0.015), even if mean VEGF levels were not modified by any training condition. Atrial natriuretic peptide (ANP) system components were not associated with increased ISIClamp during hypoxic training.
Conclusions
Physical training under hypoxic conditions could partially augment the favorable effects of exercise alone on myocellular insulin sensitivity in obese men with metabolic syndrome. Concomitant changes in VEGF might represent an underlying pathophysiological mechanism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bonora E, Formentini G, Calcaterra F, Lombardi S, Marini F, Zenari L, et al. HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabetes Care. 2002;25:1135–41.
Hanley AJ, Williams K, Stern MP, Haffner SM. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart Study. Diabetes Care. 2002;25:1177–84.
Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541–51.
Gregg EW, Chen H, Wagenknecht LE, Clark JM, Delahanty LM, Bantle J, et al. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA. 2012;308:2489–96.
Yassine HN, Marchetti CM, Krishnan RK, Vrobel TR, Gonzalez F, Kirwan JP. Effects of exercise and caloric restriction on insulin resistance and cardiometabolic risk factors in older obese adults-a randomized clinical trial. J Gerontol A Biol Sci Med Sci. 2009;64:90–5.
VDB JD, VDV JHPM, DEW EAC, Bosma H, Savelberg H, Schaper NC, et al. Replacement effects of sedentary time on metabolic outcomes: The Maastricht Study. Med. Sci. Sports Exerc. 2017;49:1351–8.
Moreno-Franco B, Penalvo JL, Casasnovas Lenguas JA, Leon-Latre M. [Compliance with Physical Activity Recommendations and Associated Factors in a Cohort of Spanish Adult Workers]. Rev Esp Salud Publica. 2015;89:447–57.
Harrison RA, Roberts C, Elton PJ. Does primary care referral to an exercise programme increase physical activity one year later? A randomized controlled trial. J Public Health (Oxf). 2005;27:25–32.
Tjonna AE, Lee SJ, Rognmo O, Stolen TO, Bye A, Haram PM, et al. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118:346–54.
Hamlin MJ, Lizamore CA, Hopkins WG. The effect of natural or simulated altitude training on high-intensity intermittent running performance in team-sport athletes: a meta-analysis. Sports Med. 2018;48:431–46.
Czuba M, Wilk R, Karpinski J, Chalimoniuk M, Zajac A, Langfort J. Intermittent hypoxic training improves anaerobic performance in competitive swimmers when implemented into a direct competition mesocycle. PLoS ONE. 2017;12:e0180380.
Haufe S, Wiesner S, Engeli S, Luft FC, Jordan J. Influences of normobaric hypoxia training on metabolic risk markers in human subjects. Med. Sci. Sports Exerc. 2008;40:1939–44.
Wiesner S, Haufe S, Engeli S, Mutschler H, Haas U, Luft FC, et al. Influences of normobaric hypoxia training on physical fitness and metabolic risk markers in overweight to obese subjects. Obesity. 2010;18:116–20.
Klug L, Mahler A, Rakova N, Mai K, Schulz-Menger J, Rahn G, et al. Normobaric hypoxic conditioning in men with metabolic syndrome. Physiol Rep. 2018;6:e13949.
Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–78.
Ameln H, Gustafsson T, Sundberg CJ, Okamoto K, Jansson E, Poellinger L, et al. Physiological activation of hypoxia inducible factor-1 in human skeletal muscle. FASEB J. 2005;19:1009–11.
Lolmede K, Durand de Saint Front V, Galitzky J, Lafontan M, Bouloumie A. Effects of hypoxia on the expression of proangiogenic factors in differentiated 3T3-F442A adipocytes. Int J Obes Relat Metab Disord. 2003;27:1187–95.
Gustafsson T. Vascular remodelling in human skeletal muscle. Biochem Soc Trans. 2011;39:1628–32.
Mandroukas A, Metaxas TI, Heller J, Vamvakoudis E, Christoulas K, Riganas CS, et al. The effect of different exercise-testing protocols on atrial natriuretic peptide. Clin Physiol Funct Imaging. 2011;31:5–10.
da Silveira Campos RM, Damaso AR, Masquio DCL, Duarte FO, Sene-Fiorese M, Aquino AE Jr., et al. The effects of exercise training associated with low-level laser therapy on biomarkers of adipose tissue transdifferentiation in obese women. Lasers Med Sci. 2018;33:1245–54.
Zhang QL, Cui BR, Li HY, Li P, Hong L, Liu LP, et al. MAPK and PI3K pathways regulate hypoxia-induced atrial natriuretic peptide secretion by controlling HIF-1 alpha expression in beating rabbit atria. Biochem. Biophys. Res. Commun. 2013;438:507–12.
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97.
Mai K, Andres J, Bobbert T, Maser-Gluth C, Mohlig M, Bahr V, et al. Rosiglitazone decreases 11beta-hydroxysteroid dehydrogenase type 1 in subcutaneous adipose tissue. Clin Endocrinol. 2007;67:419–25.
DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–23.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Toka O, Tank J, Schachterle C, Aydin A, Maass PG, Elitok S, et al. Clinical effects of phosphodiesterase 3A mutations in inherited hypertension with brachydactyly. Hypertension. 2015;66:800–8.
Haftenberger M, Schuit AJ, Tormo MJ, Boeing H, Wareham N, Bueno-de-Mesquita HB, et al. Physical activity of subjects aged 50-64 years involved in the European Prospective Investigation into Cancer and Nutrition (EPIC). Public Health Nutr. 2002;5:1163–76.
Chobanyan-Jurgens K, Scheibe RJ, Potthast AB, Hein M, Smith A, Freund R, et al. Influences of hypoxia exercise on whole body insulin sensitivity and oxidative metabolism in older individuals. J Clin Endocrinol Metab. 2019;104:5238–48.
Brachs M, Wiegand S, Leupelt V, Ernert A, Kintscher U, Jumpertz von Schwarzenberg R, et al. ANP system activity predicts variability of fat mass reduction and insulin sensitivity during weight loss. Metabolism. 2016;65:935–43.
Gorgens SW, Benninghoff T, Eckardt K, Springer C, Chadt A, Melior A, et al. Hypoxia in combination with muscle contraction improves insulin action and glucose metabolism in human skeletal muscle via the HIF-1alpha pathway. Diabetes. 2017;66:2800–7.
Slivka DR, Heesch MW, Dumke CL, Cuddy JS, Hailes WS, Ruby BC. Human skeletal muscle mRNAResponse to a single hypoxic exercise bout. Wilderness Environ Med. 2014;25:462–5.
De Smet S, D'Hulst G, Poffe C, Van Thienen R, Berardi E, Hespel P. High-intensity interval training in hypoxia does not affect muscle HIF responses to acute hypoxia in humans. Eur J Appl Physiol. 2018;118:847–62.
Lundby C, Gassmann M, Pilegaard H. Regular endurance training reduces the exercise induced HIF-1alpha and HIF-2alpha mRNA expression in human skeletal muscle in normoxic conditions. Eur J Appl Physiol. 2006;96:363–9.
Lindholm ME, Rundqvist H. Skeletal muscle hypoxia-inducible factor-1 and exercise. Exp Physiol. 2016;101:28–32.
Trayhurn P, Wang B, Wood IS. Hypoxia and the endocrine and signalling role of white adipose tissue. Arch Physiol Biochem. 2008;114:267–76.
Wagner H, Fischer H, Degerblad M, Alvarsson M, Gustafsson T. Improvement of insulin sensitivity in response to exercise training in type 2 diabetes mellitus is associated with vascular endothelial growth factor A expression. Diab Vasc Dis Res. 2016;13:361–6.
Solomon TP, Haus JM, Li Y, Kirwan JP. Progressive hyperglycemia across the glucose tolerance continuum in older obese adults is related to skeletal muscle capillarization and nitric oxide bioavailability. J Clin Endocrinol Metab. 2011;96:1377–84.
Schlueter N, de SA, Willmes DM, Spranger J, Jordan J, Birkenfeld AL. Metabolic actions of natriuretic peptides and therapeutic potential in the metabolic syndrome. Pharmacol Ther. 2014;144:12–27.
Sun JZ, Oparil S, Lucchesi P, Thompson JA, Chen YF. Tyrosine kinase receptor activation inhibits NPR-C in lung arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L155–63.
Rudvik A, Mansson M. Evaluation of surrogate measures of insulin sensitivity - correlation with gold standard is not enough. BMC Med Res Methodol. 2018;18:64.
Acknowledgements
We thank Gabriele Rahn, N. Huckauf, and C. Kalischke for excellent technical assistance.
Funding
This research was supported by the Deutsche Forschungsgemeinschaft (LU 435/13-1). KM was supported by the German Diabetes Society (DDG) and the German Ministry for Education and Research (BMBF) by support of the Berlin Institute of Health (BIH).
Author information
Authors and Affiliations
Contributions
NR, MB, LK, AM, TB, and JSM researched data. KM researched data and was primarily responsible for writing the manuscript. TB, MB, SP, AM, LK, JS, and FCL contributed to the discussion and reviewed/edited the manuscript. FCL conceived the study and obtained funding.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
The study protocols were approved by the Institutional Review Board of the Charité Medical School (EA1/140/12) and all subjects gave written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Mai, K., Klug, L., Rakova, N. et al. Hypoxia and exercise interactions on skeletal muscle insulin sensitivity in obese subjects with metabolic syndrome: results of a randomized controlled trial. Int J Obes 44, 1119–1128 (2020). https://doi.org/10.1038/s41366-019-0504-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-019-0504-z
This article is cited by
-
Anaerobic threshold using sweat lactate sensor under hypoxia
Scientific Reports (2023)
-
Post-exercise cardiac autonomic and cardiovascular responses to heart rate-matched and work rate-matched hypoxic exercise
European Journal of Applied Physiology (2021)