Abstract
Background
The murine model of high fat diet (HFD)-induced obesity is characterized by an increment of intestinal permeability, secondary to an impairment of mucosal epithelial barrier and enteric inflammation, followed by morphofunctional rearrangement of the enteric nervous system. The present study investigated the involvement of abdominal macrophages in the mechanisms underlying the development of enteric dysmotility associated with obesity.
Methods
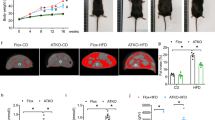
Wild type C57BL/6J mice were fed with HFD (60% kcal from fat) or normocaloric diet (NCD, 18% kcal from fat) for 8 weeks. Groups of mice fed with NCD or HFD were treated with clodronate encapsulated into liposomes to deplete abdominal macrophages. Tachykininergic contractions, elicited by electrical stimulation or exogenous substance P (SP), were recorded in vitro from longitudinal muscle colonic preparations. Substance P distribution was examined by confocal immunohistochemistry. The density of macrophages in the colonic wall was examined by immunohistochemical analysis. Malondialdehyde (MDA, colorimetric assay) and IL-1β (ELISA assay) levels were also evaluated.
Results
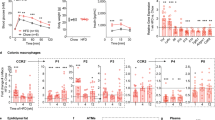
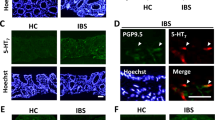
MDA and IL-1β levels were increased in colonic tissues from HFD-treated animals. In colonic preparations, electrically evoked tachykininergic contractions were enhanced in HFD mice. Immunohistochemistry displayed an increase in substance P immunoreactivity in myenteric ganglia, as well as in the muscular layers of colonic cryosections from obese mice. Macrophage depletion in HFD mice was associated with a significant reduction of colonic inflammation. In addition, the decrease in macrophage density attenuated the morphofunctional alterations of tachykininergic pathways observed in obese mice.
Conclusion
Obesity elicited by HFD determines a condition of colonic inflammation, followed by a marked rearrangement of motor excitatory tachykininergic enteric nerves. Macrophage depletion counteracted the morphofunctional changes of colonic neuromuscular compartment, suggesting a critical role for these immune cells in the onset of enteric dysmotility associated with obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–7.
Carolan E, Tobin LM, Mangan BA, Corrigan M, Gaoatswe G, Byrne G, et al. Altered distribution and increased IL-17 production by mucosal-associated invariant T cells in adult and childhood obesity. J Immunol. 2015;194:5775–80.
Sun S, Ji Y, Kersten S, Qi L. Mechanisms of inflammatory responses in obese adipose tissue. Annu Rev Nutr. 2012;32:261–86.
Lam YY, Ha CW, Campbell CR, Mitchell AJ, Dinudom A, Oscarsson J, et al. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS ONE. 2012;7:e34233.
Raybould HE. Gut microbiota, epithelial function and derangements in obesity. J Physiol. 2012;590:441–6.
Stenman LK, Holma R, Korpela R. High-fat-induced intestinal permeability dysfunction associated with altered fecal bile acids. World J Gastroenterol. 2012;18:923–9.
Bertrand RL, Senadheera S, Markus I, Liu L, Howitt L, Chen H, et al. A Western diet increases serotonin availability in rat small intestine. Endocrinology. 2011;152:36–47.
Nezami BG, Mwangi SM, Lee JE, Jeppsson S, Anitha M, Yarandi SS, et al. MicroRNA 375 mediates palmitate-induced enteric neuronal damage and high-fat diet-induced delayed intestinal transit in mice. Gastroenterology. 2014;146:473–83.
Fysekidis M, Bouchoucha M, Bihan H, Reach G, Benamouzig R, Catheline JM. Prevalence and co-occurrence of upper and lower functional gastrointestinal symptoms in patients eligible for bariatric surgery. Obes Surg. 2012;22:403–10.
Le Pluart D, Sabate JM, Bouchoucha M, Hercberg S, Benamouzig R, Julia C. Functional gastrointestinal disorders in 35,447 adults and their association with body mass index. Aliment Pharmacol Ther. 2015;41:758–67.
Ridha M, Nourse SE, Selamet Tierney ES. Pediatric interventions using noninvasive vascular health indices. Hypertension. 2015;65:949–55.
Di Giovangiulio M, Verheijden S, Bosmans G, Stakenborg N, Boeckxstaens GE, Matteoli G. The neuromodulation of the intestinal immune system and its relevance in inflammatory bowel disease. Front Immunol. 2015;6:590.
Muller PA, Koscso B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–13.
Lomax AE, Fernandez E, Sharkey KA. Plasticity of the enteric nervous system during intestinal inflammation. Neurogastroenterol Motil. 2005;17:4–15.
Vasina V, Barbara G, Talamonti L, Stanghellini V, Corinaldesi R, Tonini M, et al. Enteric neuroplasticity evoked by inflammation. Auton Neurosci: Basic Clin. 2006;126-7:264–72.
Neunlist M, Aubert P, Toquet C, Oreshkova T, Barouk J, Lehur PA, et al. Changes in chemical coding of myenteric neurones in ulcerative colitis. Gut. 2003;52:84–90.
ter Beek WP, Biemond I, Muller ES, van den Berg M, Lamers CB. Substance P receptor expression in patients with inflammatory bowel disease. Determination by three different techniques, i.e., storage phosphor autoradiography, RT-PCR and immunohistochemistry. Neuropeptides. 2007;41:301–6.
Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93.
Siegmund B, Lehr HA, Fantuzzi G, Dinarello CA. IL-1 beta -converting enzyme (caspase-1) in intestinal inflammation. Proc Natl Acad Sci USA. 2001;98:13249–54.
Antonioli L, Fornai M, Colucci R, Awwad O, Ghisu N, Tuccori M, et al. The blockade of adenosine deaminase ameliorates chronic experimental colitis through the recruitment of adenosine A2A and A3 receptors. J Pharmacol Exp Ther. 2010;335:434–42.
Antonioli L, Pellegrini C, Fornai M, Tirotta E, Gentile D, Benvenuti L et al. Colonic motor dysfunctions in a mouse model of high-fat diet-induced obesity: an involvement of A2B adenosine receptors. Purinergic Signal. 2017;13:497–510.
Mowat AM, Bain CC. Mucosal macrophages in intestinal homeostasis and inflammation. J Innate Immun. 2011;3:550–64.
Caputi V, Marsilio I, Filpa V, Cerantola S, Orso G, Bistoletti M, et al. Antibiotic-induced dysbiosis of the microbiota impairs gut neuromuscular function in juvenile mice. Br J Pharmacol. 2017;174:3623–39.
Orso G, Martinuzzi A, Rossetto MG, Sartori E, Feany M, Daga A. Disease-related phenotypes in a Drosophila model of hereditary spastic paraplegia are ameliorated by treatment with vinblastine. J Clin Invest. 2005;115:3026–34.
Engel MA, Leffler A, Niedermirtl F, Babes A, Zimmermann K, Filipovic MR, et al. TRPA1 and substance P mediate colitis in mice. Gastroenterology. 2011;141:1346–58.
Kim KA, Gu W, Lee IA, Joh EH, Kim DH. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS ONE. 2012;7:e47713.
Laurila A, Cole SP, Merat S, Obonyo M, Palinski W, Fierer J, et al. High-fat, high-cholesterol diet increases the incidence of gastritis in LDL receptor-negative mice. Arterioscler Thromb Vasc Biol. 2001;21:991–6.
Kolbus D, Ramos OH, Berg KE, Persson J, Wigren M, Bjorkbacka H, et al. CD8+T cell activation predominate early immune responses to hypercholesterolemia in Apoe(-)(/)(-) mice. BMC Immunol. 2010;11:58.
Ding S, Lund PK. Role of intestinal inflammation as an early event in obesity and insulin resistance. Curr Opin Clin Nutr Metab Care. 2011;14:328–33.
Gulhane M, Murray L, Lourie R, Tong H, Sheng YH, Wang R, et al. High fat diets induce colonic epithelial cell stress and inflammation that is reversed by IL-22. Sci Rep. 2016;6:28990.
Lam YY, Ha CW, Hoffmann JM, Oscarsson J, Dinudom A, Mather TJ, et al. Effects of dietary fat profile on gut permeability and microbiota and their relationships with metabolic changes in mice. Obesity. 2015;23:1429–39.
Lim SM, Jeong JJ, Woo KH, Han MJ, Kim DH. Lactobacillus sakei OK67 ameliorates high-fat diet-induced blood glucose intolerance and obesity in mice by inhibiting gut microbiota lipopolysaccharide production and inducing colon tight junction protein expression. Nutr Res. 2016;36:337–48.
Anitha M, Reichardt F, Tabatabavakili S, Nezami BG, Chassaing B, Mwangi S, et al. Intestinal dysbiosis contributes to the delayed gastrointestinal transit in high-fat diet fed mice. Cell Mol Gastroenterol Hepatol. 2016;2:328–39.
Mushref MA, Srinivasan S. Effect of high fat-diet and obesity on gastrointestinal motility. Ann Transl Med. 2013;1:14.
Antonioli MF L, Pellegrini C, Tirotta E, Gentile D, Benvenuti L, Caputi V, et al. Colonic dysmotility associated with high fat diet-induced obesity: role of the enteric glia. Gastroenterology. 2017;152:S180
Fu XY, Li Z, Zhang N, Yu HT, Wang SR, Liu JR. Effects of gastrointestinal motility on obesity. Nutr Metab. 2014;11:3.
Johnson AR, Milner JJ, Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev. 2012;249:218–38.
Ghigliotti G, Barisione C, Garibaldi S, Fabbi P, Brunelli C, Spallarossa P, et al. Adipose tissue immune response: novel triggers and consequences for chronic inflammatory conditions. Inflammation. 2014;37:1337–53.
Bing C. Is interleukin-1beta a culprit in macrophage-adipocyte crosstalk in obesity? Adipocyte. 2015;4:149–52.
Khan WI, Collins SM. Gut motor function: immunological control in enteric infection and inflammation. Clin Exp Immunol. 2006;143:389–97.
Margolis KG, Gershon MD. Neuropeptides and inflammatory bowel disease. Curr Opin Gastroenterol. 2009;25:503–11.
Mikkelsen HB. Interstitial cells of Cajal, macrophages and mast cells in the gut musculature: morphology, distribution, spatial and possible functional interactions. J Cell Mol Med. 2010;14:818–32.
Luck H, Tsai S, Chung J, Clemente-Casares X, Ghazarian M, Revelo XS, et al. Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab. 2015;21:527–42.
Bu L, Gao M, Qu S, Liu D. Intraperitoneal injection of clodronate liposomes eliminates visceral adipose macrophages and blocks high-fat diet-induced weight gain and development of insulin resistance. AAPS J. 2013;15:1001–11.
Stienstra R, Saudale F, Duval C, Keshtkar S, Groener JE, van Rooijen N, et al. Kupffer cells promote hepatic steatosis via interleukin-1beta-dependent suppression of peroxisome proliferator-activated receptor alpha activity. Hepatology. 2010;51:511–22.
Negrin KA, Roth Flach RJ, DiStefano MT, Matevossian A, Friedline RH, Jung D, et al. IL-1 signaling in obesity-induced hepatic lipogenesis and steatosis. PLoS ONE. 2014;9:e107265.
Author contributions
LA, RC, MF, CP, GH and CB designed the study, DG, MCG, VC, GO, NB, CSe, CI, BC, and ZHN collected and analyzed data, LA, RC, MF, CSc, CP, GH, and CB wrote the manuscript. All authors discussed the results and commented on the manuscript.
Funding
This work was supported by PRA_2016_20 granted by the University of Pisa and by the supported by U.S. National Institutes of Health (NIH) NIH 1R01DK113790 grant. The present work was funded by University of Padova (Italy) PRID 2016 to R.C, by University of Padova (Italy) Assegno di Ricerca 2016 and by San Camillo Hospital, Treviso (Italy) Grant 2015 to M.C.G.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Antonioli, L., Caputi, V., Fornai, M. et al. Interplay between colonic inflammation and tachykininergic pathways in the onset of colonic dysmotility in a mouse model of diet-induced obesity. Int J Obes 43, 331–343 (2019). https://doi.org/10.1038/s41366-018-0166-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0166-2