Abstract
Background
The optimal approach for reporting reintubation rates in extremely preterm infants is unknown. This study aims to longitudinally describe patterns of reintubation in this population over a broad range of observation windows following extubation.
Methods
Timing and reasons for reintubation following a first planned extubation were collected from infants with birth weight ≤1,250 g. An algorithm was generated to discriminate between reintubations attributable to respiratory and non-respiratory causes. Frequency and cumulative distribution curves were constructed for each category using 24 h intervals. The ability of observation windows to capture respiratory-related reintubations while limiting non-respiratory reasons was assessed using a receiver operating characteristic curve.
Results
Out of 194 infants, 91 (47%) were reintubated during hospitalization; 68% for respiratory and 32% for non-respiratory reasons. Respiratory-related reintubation rates steadily increased from 0 to 14 days post-extubation before reaching a plateau. In contrast, non-respiratory reintubations were negligible in the first post-extubation week, but became predominant after 14 days. An observation window of 7 days captured 77% of respiratory-related reintubations while only including 14% of non-respiratory cases.
Conclusion
Reintubation patterns are highly variable and affected by the reasons for reintubation and observation window used. Ideally, reintubation rates should be reported using a cumulative distribution curve over time.
Similar content being viewed by others
Main
With the increasing use of early non-invasive respiratory strategies, mechanical ventilation (MV) is becoming reserved for the most immature infants with severe respiratory disease (1, 2). In an effort to avoid the complications associated with MV (3), prompt weaning and reduction of MV duration remain the ultimate goal. Unfortunately, as many as two-thirds of extremely low birth weight (ELBW) infants (birth weight ≤1,000 g) fail the initial extubation attempt and require reintubation during hospitalization (4, 5). Reintubation not only prolongs MV duration but has also been independently associated with morbidities and mortality (4, 5, 6). For these reasons, respiratory-related studies commonly investigate strategies that can reduce rates of reintubation in these infants (7).
Reported rates of reintubation are typically defined as the proportion of infants reintubated within a prespecified time after extubation. In general, studies aim to select an observation window that captures the majority of reintubations attributable to respiratory failure while minimizing the inclusion of reintubations caused by non-respiratory events. However, since the optimal window of observation is unknown, variable ranges have been used. A recent systematic review on this subject noted that observation windows ranged anywhere from 12 h to 7 days after extubation, without distinction between the causes of reintubation (8). Interestingly, among ELBW infants, reintubation rates significantly increased as a function of the selected observation window, without reaching a plateau by 7 days (8). Thus, the aim of this study was to longitudinally evaluate patterns of reintubation over a broad range of observation windows in extremely preterm infants, and determine the optimal approach for reporting reintubation rates in this population.
Methods
Study design
This work is a substudy of APEX, an ongoing prospective multicenter observational trial aimed at developing an automated prediction tool of extubation readiness in extremely preterm infants (9). The study was conducted in five neonatal intensive care units at the Royal Victoria Hospital, Jewish General Hospital, and Montreal Children’s Hospital (Montreal, QC, Canada); Detroit Medical Centre (Detroit, MI) and Women and Infants Hospital (Providence, Rhode Island). Ethics approval was received from each institution’s Research Ethics Board and informed parental consent was obtained.
Study population
Infants with birth weights ≤1,250 g and requiring MV were eligible. Only infants who underwent a planned extubation were included; an extubation was considered planned when the infant was deemed ready by the responsible clinician and extubation occurred under controlled conditions (9). Infants with major congenital anomalies, congenital heart disease, vasopressor or sedative use at extubation, extubations from high-frequency ventilation, and unplanned extubations (i.e., endotracheal tube dislodgement or obstruction) were excluded. All infants enrolled were followed from birth until death, discharge, or transfer from the neonatal intensive care unit. All decisions related to weaning from MV, timing of extubation, post-extubation respiratory support and reintubation were made by the responsible clinician and not influenced by the study team.
Data collection
As part of APEX, a comprehensive database of clinical variables was prospectively collected. An automated quality control and validation process was developed and applied to all clinical files to correct for any transcription errors or outliers, as described elsewhere (10). The present study dealt with the subset of infants that were reintubated at any time point after their first planned extubation, and focused on the following variables:
-
a
Demographic information: Gestational age, birth weight, sex, antenatal steroid administration, caesarian section, 5-min APGAR score, use of surfactant, and use of caffeine.
-
b
At the time of first planned extubation: Postmenstrual age, weight, postnatal age, pre- and post-extubation ventilatory settings, and blood gases.
-
c
At the time of reintubation: The timing and reasons leading to reintubation, along with the type of respiratory support needed, were ascertained from the medical chart and/or directly from the clinical team. Data abstractors were instructed to check one or more of the following preselected categorical responses to indicate the reason(s) used by the medical team to proceed with reintubation: (1) apneas and bradycardias, (2) increased work of breathing (WOB), (3) respiratory acidosis, (4) increased O2 needs, (5) upper airway obstruction, and (6) other causes. The latter were defined as any other reason(s) or diagnose(s) that could explain the reintubation, such as infections (bacteremia, bronchiolitis, urinary tract infection), gastrointestinal complications (necrotizing enterocolitis, perforation, volvulus), or elective procedures, and were documented in a free-form text field in the data collection form.
Data analysis
All analyses were performed using MATLAB (R2016a, The MathWorks, Natick, MA, USA). Continuous and categorical variables were described using medians (interquartile range) and counts (percentages), respectively. The timing of each reintubation was computed as the date and time of reintubation minus the date and time of first planned extubation, and grouped into 24 h bins to create a broad range of observation windows. The proportion of infants reintubated for each of the six categorical reasons for reintubation was determined. The free-text responses were analyzed using an automated classification algorithm (developed by W.S. and R.E.K.), which was able to detect keywords belonging to three additional categories of reintubation: infections (e.g., “sepsis”, “bacteremia”, “cons”, “bronchiolitis”, “uti”), gastrointestinal complications (e.g., “necrotizing”, “colitis”, “nec”, “volvulus”, “perf”), or elective procedures (e.g., “planned”, “elective”, “operation”, “surgery”, “procedure”). Infants reintubated for any of these three reasons were classified as non-respiratory; infants who were reintubated due to apneas and bradycardias, increased WOB, respiratory acidosis, and/or increased O2 needs but without any other detectable etiology, were classified as respiratory reintubations. To verify that the algorithm was accurate, a manual review of the free-text responses in the data collection forms was performed on a subset of 74 reintubated infants. The manual inspection confirmed that all reviewed infants were accurately classified into their respective categories by the algorithm.
Since the main objective of this study was to determine the proportion of infants reintubated and reasons for reintubation over a broad range of observation windows following the first planned extubation, the following sequence of analyses was conducted:
-
1)
A frequency distribution curve was plotted to describe the number of infants reintubated per 24 h bin.
-
2)
Cumulative distribution curves were constructed to describe the reintubation rates for respiratory and non-respiratory reintubations as a function of the observation window used.
-
3)
One-sample proportion tests were used to compare the relative probabilities of reintubation due to respiratory vs. non-respiratory reasons during three periods: 0–7, 8–14, and >14 days after the first planned extubation. A P value <0.05 was considered statistically significant, and 95% confidence intervals (CIs) were computed.
-
4)
The trade-off between the ability of various observation windows to capture all respiratory reintubations (sensitivity) while limiting non-respiratory reasons (specificity) was evaluated using a receiver operating characteristic curve.
-
5)
A subgroup analysis was performed on infants with birth weights ≤1,000 g to identify their cumulative rates of respiratory and non-respiratory reintubation over time.
Results
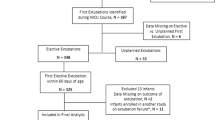
From September 2013 to June 2016, a total of 547 infants met the eligibility criteria; 270 were consented and 194 were included in this study (Figure 1). A total of 91 infants (47%) were reintubated at some time after their first planned extubation. Their demographics and pre-extubation characteristics are shown in Table 1. The majority (85%) of infants were reintubated from non-synchronized nasal intermittent positive pressure ventilation, and the most common reason for reintubation was apneas and bradycardias (65%). No infant was reintubated for upper airway obstruction. An infection (bacteremia, urinary tract infection, or bronchiolitis) was reported as a contributing cause in 17 infants (19%), whereas gastrointestinal complications (necrotizing enterocolitis, intestinal perforation, or volvulus) and elective procedures accounted for 10 (11%) and 5 (5%) cases, respectively. Three infants had coexisting diagnoses of infection and necrotizing enterocolitis upon reintubation.
The automated classification algorithm identified 62 respiratory and 29 non-respiratory reintubations. The frequency distribution of reintubations over time is presented in Figure 2. The highest peak of reintubations occurred within 24 h from extubation, representing 21% of all reintubations. A subsequent peak occurred between 25 and 168 h after extubation, accounting for an additional 36% of all reintubations. A similar distribution pattern was observed for the subset of respiratory reintubations, with the proportion of reintubations being higher at both 24 h (29%) and between 25 and 168 h (48%). Respiratory reintubations in the first 24 h were primarily related to one or more of the following: increased WOB (61%), apneas and bradycardias (44%), and increased O2 needs (44%). Respiratory reintubations beyond 24 h were mostly due to apneas and bradycardias (73%), and to a lesser extent due to increased WOB and O2 needs (32% each).
The cumulative distribution curve of respiratory and non-respiratory reintubation rates over time is displayed in Figure 3. Respiratory-related reintubation rates steadily increased as the observation window was extended from 0 to 14 days post-extubation, after which a plateau was finally reached. The rates of respiratory-related reintubations were 15% at 72 h, 25% at 7 days, 29% at 14 days, and 32% at discharge. In contrast, rates of non-respiratory reintubations were only 2% in the first 7 days after extubation and increased gradually, reaching 15% at discharge.
The relative probabilities of respiratory and non-respiratory reintubations for three different time periods after extubation are shown in Figure 4. Infants reintubated within 7 days from extubation had a significantly greater probability of respiratory-related reintubation (P<0.001, 95% CI: 85–100%), whereas those reintubated beyond 14 days had a significantly greater probability of non-respiratory reintubation (P=0.007, 95% CI: 5–39%). Reintubations between 8 and 14 days were equally attributed to respiratory and non-respiratory causes (P=0.62, 95% CI: 32–81%).
Finally, Figure 5 shows a receiver operating characteristic curve that plots the proportion of respiratory reintubations (y-axis) and non-respiratory reintubations (x-axis), captured by various observation windows. Time windows of 72 h, 7 days, 10 days, and 14 days captured 47%, 77%, 85%, and 92% of respiratory reintubations, at the expense of also detecting 3%, 14%, 21%, and 38% of non-respiratory reintubations, respectively.
Receiver operating characteristics curve of the optimal observation window for detecting respiratory-related reintubations. Red circles represent the sensitivity (proportion of respiratory reintubations detected) and specificity (proportion of non-respiratory reintubations detected) of different observation windows (grouped into 24 h intervals) between 24 h and 21 days post-extubation.
For the subgroup analysis, a total of 79 out of 137 ELBW infants (58%) were reintubated at any time point after their first planned extubation, 55 of which were respiratory (70%) and 24 (30%) non-respiratory. The cumulative distribution curve of respiratory and non-respiratory reintubation rates for ELBW infants is available in Supplementary Figure S1 online.
Discussion
This paper describes, for the first time, the patterns of reintubation in extremely preterm infants over a broad range of observation windows following extubation. In our cohort, nearly half the infants were reintubated at some time after their first planned extubation; two-thirds for respiratory reasons and one-third for non-respiratory causes. Reintubation rates increased consistently as the observation window was extended beyond 7 days, with differences between respiratory and non-respiratory cases. Furthermore, a comprehensive summary of the sensitivity and specificity of different observation windows at capturing respiratory reintubations without overdetecting non-respiratory causes is provided. Altogether, these findings improve the knowledge concerning reintubation of this vulnerable population and add a valuable framework for standardizing its reporting in the scientific literature.
We report that 47% of infants ≤1,250 g and 58% of infants ≤1,000 g were reintubated at least once during hospitalization, a finding consistent with results of other large cohort studies (4, 11). However, these reintubation rates are much higher than those reported for adult (10%) and children (6%) patients (12, 13). Given the morbidities associated with reintubation and resumption of MV, clinicians and investigators alike are increasingly searching for strategies that can reduce rates of reintubation in this population (7). Unfortunately, a heretofore, these reintubation rates have not been reported in a standardized manner, making comparisons between studies quite difficult (14). For instance, in a recent meta-analysis of interventions to improve the extubation success, pooled studies used variable durations of observation (ranging from 24 h to 7 days) and included infants with varying degrees of prematurity (all gestational ages <37 weeks) (7). Our data suggest that reintubation rates changed considerably as a function of the observation window used and population studied. To illustrate, reintubation rates in our cohort would have been reported as 10% after 24 h of observation, 16% after 72 h, or 27% after 7 days, whereas limiting the study to ELBW infants could have resulted in rates of 13%, 21%, or 34% for the same time points. These results support the concerns raised previously in a systematic review where reintubation rates among ELBW infants significantly increased over time but did not reach a plateau at 7 days post-extubation, which was not the case in larger infants (8). Therefore, in the extremely preterm population, comparison between studies that use different populations or definitions may potentially lead to inaccurate or misleading conclusions.
In studies investigating the outcome of extubation success or failure, the selected observation window has an important impact on the types of reintubation included. According to a recent international survey, most clinicians reported that extubation failure should be defined as the need for reintubation within 24–72 h after extubation (15). The choice of a short observation window reflects the assumption that most reintubations caused by respiratory disease would occur in this time frame (5). Indeed, a recent secondary analysis of the Surfactant, Positive Pressure, and Oxygenation Randomized Trial (SUPPORT) used a cutoff of 5 days to define extubation success based on the rationale that most reintubations during that window occurred within 48 h after extubation (5). However, limiting the observation period to 5 days failed to account for an additional 243 infants who failed beyond that cutoff, hence excluding 39% of all reintubations from their analysis. In our study, we similarly observed that only a small proportion of reintubations attributable to respiratory disease actually occurred in the first few days after extubation. In fact, limiting the observation windows to 24 h, 72 h, and 7 days systematically underreported the true number of respiratory reintubations, failing to capture more than 70%, 50%, and 20% of actual events, respectively. Such findings call into question the interpretation of studies testing interventions to prevent reintubation, since limiting the reports to short observation windows may provide a falsely reassuring estimate of the effectiveness of the intervention.
We found it quite interesting that a considerable number of reintubations that occurred between 72 h and 14 days after extubation had no identifiable cause other than respiratory-related symptoms (apneas and bradycardias, increased WOB, respiratory acidosis, and/or increased oxygen needs). There are several plausible explanations for the need for extended observation windows to capture these respiratory-related cases. First, with the increased use of non-invasive ventilation following extubation, reintubation may be delayed rather than actually prevented. This phenomenon has actually been described in the adult population, whereby the increased use of non-invasive respiratory support has shifted the window of observation from the traditional 48 to 96 h post-extubation (12, 16). Indeed, in our cohort, although infants were extubated to continuous positive airway pressure or nasal intermittent positive pressure ventilation equally often, the majority had escalated to nasal intermittent positive pressure ventilation by the time they were reintubated. This practice stems from meta-analyses demonstrating that nasal intermittent positive pressure ventilation may be superior to continuous positive airway pressure in reducing reintubation rates among preterm infants (7, 17). However, most studies used a cutoff of 48–72 h for their definitions, making it unclear as to whether extended windows of observation would have resulted in sustained benefits or not. Second, there is currently little evidence as to what constitutes a physiologically and clinically relevant definition of non-invasive support failure. As a result, the decision to reintubate is highly subjective and generally based on clinicians’ interpretation of apnea severity and/or frequency, oxygen requirements, WOB, and gas exchange (8). A recent international survey reported that only 10% of respondents reintubated infants on the basis of standardized guidelines (15). Thus, there is significant variability within and between units regarding tolerance thresholds for reintubation. However, given the concerns about reinstituting MV, it is conceivable that some clinicians would preferentially attempt to postpone reintubation as much as possible. Third, extremely preterm infants are reintubated due to a multitude of respiratory-related reasons, including lung atelectasis, inflammation, pulmonary edema, high airway resistance, and suboptimal respiratory drive. Unfortunately, indications currently used by clinicians to justify reintubation (such as increased WOB or apneas) lack specificity to distinguish between those respiratory causes. In our cohort, we observed two distinct patterns of respiratory-related reintubations, one within 24 h and another between 25 and 168 h after extubation, raising the hypothesis that the various respiratory disease processes leading to reintubation may actually manifest at variable time frames following extubation.
It is clear from the present analysis that extending the observation window will capture all respiratory reintubations, but at the cost of including reintubations that are unrelated to respiratory failure. In our study, reintubations due to non-respiratory causes were negligible in the first week after extubation, but became much more frequent after 14 days. The receiver operating characteristic curve on Figure 5 suggests that using observation windows between 7 and 14 days would offer the optimal trade-off between sensitivity and specificity for detecting respiratory reintubations.
Our study had certain limitations. The analysis was only performed for reintubations following the first planned extubation, and therefore cannot be extrapolated to those that occurred after repeated or unplanned extubations. Moreover, it is always possible that some reintubations may have been misclassified as respiratory or non-respiratory by the medical team (i.e., misdiagnosis), during the data extraction process or by the classification algorithm. Finally, due to the wide variability in respiratory care practices and comorbidities (e.g., postnatal sepsis or necrotizing enterocolitis) across neonatal intensive care units, our results may not be generalizable to all centers. Nevertheless, the study’s multicenter nature, large sample size, prospective design, thorough data quality control, and rigorous analytical methods provide new and critical information for accurately reporting and analysing patterns of reintubation in extremely preterm infants undergoing their first extubation attempt. Our findings also highlight the importance of the choice of time frame and causes of reintubation when studying this population. Future research is needed to objectively characterize the various disease processes leading to respiratory-related reintubations in the first 14 days after extubation. Furthermore, the clinical implications of reintubation at different time frames and for different reasons should be explored.
In conclusion, reintubation is a very common but complex phenomenon that occurs due to multiple reasons and at variable frequencies throughout hospitalization. As a consequence, studies reporting reintubation rates at any single time point after extubation will provide an incomplete overview of the true reintubation rates, making them difficult to interpret or compare. Future studies investigating the outcome of extubation success or failure, irrespective of the definition used, should report reintubation rates as a continuum, by presenting a cumulative distribution curve over time. Ideally, this time frame should extend to at least 7 days, since it would capture most of the cases of respiratory failure without including non-respiratory-related reintubations. If a longer window is chosen, distinction should be made between respiratory and non-respiratory reintubations. Finally, traditional statistical methods should preferentially be replaced by time-to-event analysis techniques as a means to more accurately compare the effectiveness of interventions at reducing rates of reintubations.
References
Stoll BJ, Hansen NI, Bell EF et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 2015;314:1039–1051.
Finer NN, Carlo WA, Walsh MC et al. Early CPAP versus surfactant in extremely preterm infants. N Eng J Med 2010;362:1970–1979.
Miller JD, Carlo WA . Pulmonary complications of mechanical ventilation in neonates. Clin Perinatol 2008;35:273–281.
Jensen EA, DeMauro SB, Kornhauser M, Aghai ZH, Greenspan JS, Dysart KC . Effects of multiple ventilation courses and duration of mechanical ventilation on respiratory outcomes in extremely low-birth-weight infants. JAMA Pediatr 2015;169:1011–1017.
Chawla S, Natarajan G, Shankaran S et al. Markers of successful extubation in extremely preterm infants, and morbidity after failed extubation. J Pediatr 2017;189:113–119.
Manley BJ, Doyle LW, Owen LS, Davis PG . Extubating extremely preterm infants: predictors of success and outcomes following failure. J Pediatr 2016;173:45–49.
Ferguson KN, Roberts CT, Manley BJ, Davis PG . Interventions to improve rates of successful extubation in preterm infants: a systematic review and meta-analysis. JAMA Pediatr 2017;171:165–174.
Giaccone A, Jensen E, Davis P, Schmidt B . Definitions of extubation success in very premature infants: a systematic review. Arch Dis Child Fetal Neonatal Ed 2014;99:F124–F127.
Shalish W, Kanbar LJ, Rao S et al. Prediction of extubation readiness in extremely preterm infants by the automated analysis of cardiorespiratory behavior: study protocol. BMC Pediatr 2017;17:167.
Kanbar LJ, Shalish W, Precup D, Brown K, Sant'Anna GM, Kearney RE . Automated ongoing data validation and quality control of multi-institutional studies. Conf Proc IEEE Eng Med Biol Soc 2016: 2504–2507.
Berger J, Mehta P, Bucholz E, Dziura J, Bhandari V . Impact of early extubation and reintubation on the incidence of bronchopulmonary dysplasia in neonates. Am J Perinatol 2014;31:1063–1072.
Miltiades AN, Gershengorn HB, Hua M, Kramer AA, Li G, Wunsch H . Cumulative probability and time to reintubation in U.S. ICUs. Crit Care Med 2017;45:835–842.
Kurachek SC, Newth CJ, Quasney MW et al. Extubation failure in pediatric intensive care: a multiple-center study of risk factors and outcomes. Crit Care Med 2003;31:2657–2664.
Manley BJ, Davis PG . Solving the extubation equation: successfully weaning infants born extremely preterm from mechanical ventilation. J Pediatr 2017;189:17–18.
Al-Mandari H, Shalish W, Dempsey E, Keszler M, Davis PG, Sant'Anna G . International survey on periextubation practices in extremely preterm infants. Arch Dis Child Fetal Neonatal Ed 2015;100:F428–F431.
Thille AW, Richard JC, Brochard L . The decision to extubate in the intensive care unit. Am J Respir Crit Care Med 2013;187:1294–1302.
Lemyre B, Davis PG, De Paoli AG, Kirpalani H . Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database Syst Rev 2017;2:CD003212.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
STATEMENT OF FINANCIAL SUPPORT
The project has received funding via an operational grant from the Canadian Institutes of Health Research. The funding body did not have a role in the design and collection, analysis, or interpretation of the data.
Supplementary information
Rights and permissions
About this article
Cite this article
Shalish, W., Kanbar, L., Keszler, M. et al. Patterns of reintubation in extremely preterm infants: a longitudinal cohort study. Pediatr Res 83, 969–975 (2018). https://doi.org/10.1038/pr.2017.330
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2017.330
This article is cited by
-
The Diaphragmatic Initiated Ventilatory Assist (DIVA) trial: study protocol for a randomized controlled trial comparing rates of extubation failure in extremely premature infants undergoing extubation to non-invasive neurally adjusted ventilatory assist versus non-synchronized nasal intermittent positive pressure ventilation
Trials (2024)
-
Predictors of extubation failure in newborns: a systematic review and meta-analysis
Italian Journal of Pediatrics (2023)
-
Oxygen saturation histogram classification system to evaluate response to doxapram treatment in preterm infants
Pediatric Research (2023)
-
Towards precision medicine for extubation of extremely preterm infants: is variability the spice of life?
Pediatric Research (2023)
-
Cardiorespiratory measures shortly after extubation and extubation outcomes in extremely preterm infants
Pediatric Research (2023)