Abstract
Reduced cerebral function after neonatal hypoxia-ischemia is an early indicator of hypoxic-ischemic encephalopathy. Near-infrared spectroscopy offers a clinically relevant means of detecting impaired cerebral metabolism from the measurement of the cerebral metabolic rate of oxygen (CMRO2). The purpose of this study was to determine the relationship between postinsult CMRO2 and duration of hypoxia-ischemia in piglets. Twelve piglets were subjected to randomly selected durations of hypoxia-ischemia (5-28 min) and five animals served as controls. Measurements of CMRO2 were taken before and for 24 h after hypoxia-ischemia. Histology was carried out in nine piglets (six insults, three controls) to estimate brain injury. In the first 4 h after the insult, average CMRO2 of the insult group was significantly depressed (33 ± 3% reduction compared with controls) and by 8 h, a significant correlation developed, which persisted for the remainder of the study, between CMRO2 and the duration of ischemia. Histologic staining suggested little brain damage resulted from shorter insult durations and considerable damage from more prolonged insults. This study demonstrated that near-infrared spectroscopy could detect early changes in CMRO2 after hypoxia-ischemia for a range of insult severities and CMRO2 could be used to distinguish insult severity by 8 h after the insult.
Similar content being viewed by others
Main
Perinatal hypoxia-ischemia continues to be a significant cause of neurodevelopmental impairment and disability in the term infant. Results from animal and newborn human studies suggest a significant portion of hypoxic-ischemic brain damage can be delayed by 6-72 h after resuscitation (1). Long-term outcome could be improved if mechanisms leading to delayed cell death could be suppressed during the latent period between insult and injury—a period termed the “therapeutic window” (2). The exact duration of the therapeutic window is unknown; however, the success rate of treatment strategies depends on early administration. Unfortunately, the clinical signs of perinatal hypoxia-ischemia can be subtle and there are as yet no proven methods capable of reliably and accurately assessing the severity of injury within the confines of the therapeutic window (2).
For nearly 30 y it has been known that cerebral ischemia can cause long-term depression of cerebral metabolism despite resuscitation (3). Studies conducted in neonatal lambs demonstrated that a reduction in cerebral energy metabolism can be seen within 4 h after resuscitation from hypoxia-ischemia (4,5) and metabolic impairment has been similarly observed in newborns within 18 h of birth by proton magnetic resonance spectroscopy (6), while delayed energy failure, as quantified by phosphorous magnetic resonance spectroscopy, can be used to predict neurologic outcome (7). The results of these studies suggest that a noninvasive measure of the cerebral metabolic rate of oxygen (CMRO2) may be of particular interest to the clinical prognosis of hypoxia-ischemia.
Recent technical advances have made near-infrared spectroscopy (NIRS) a promising tool for assessing brain function at the bedside of critically ill newborns. With continuous-wave, broadband-spectrum NIRS, the absolute concentration of cerebral deoxy-Hb ([HHb]) (8) and cerebral blood flow (CBF) (9) can be accurately determined. By combining these measurements, we have developed a NIRS system capable of rapidly measuring CMRO2 and have validated it in newborn piglets over a range of metabolic states (10,11).
The NIRS measurement of CMRO2 was also validated in a study that monitored piglets for 6 h after resuscitation from 30 min of hypoxia-ischemia (12). An immediate and persistent 25% reduction in CMRO2 was observed after the insult, suggesting NIRS has promise as an indicator of hypoxia-ischemia within the therapeutic window. However, the sensitivity of the NIRS measurement to insult severity was not investigated. The purpose of the present study was to grade the duration of hypoxia-ischemia in piglets to determine the relationship between posthypoxia-ischemia measures of CMRO2 and insult severity, as quantified by the duration of ischemia.
MATERIALS AND METHODS
Animal preparation.
The study was approved by the Animal Use Subcommittee of the Canadian Council on Animal Care at the University of Western Ontario. Newborn Duroc piglets (n = 17) were delivered from a local supplier on the morning of the experiment. Anesthesia was induced and surgery was conducted using 3% isoflurane. The surgical procedure has been presented in detail elsewhere (12). Briefly, piglets were tracheotomized and mechanically ventilated. Vascular occluders (In Vivo Metric, Healdsberg, CA) were placed around both carotid arteries just proximal to the carotid bifurcation. Catheters were inserted into an ear and an abdominal vein for injection of the NIRS-sensitive, intravascular contrast agent indocyanine green (ICG) and for glucose infusion, respectively. Another catheter was inserted into a femoral artery for continuous monitoring of blood pressure and for collection of arterial blood samples for gas and glucose determinations. Isoflurane was reduced to 1.75% after surgery and maintained for the entire experiment.
Experimental procedures.
The piglets were randomly divided into two groups: an insult group (n = 12) and a sham-operated control group (n = 5). After 1 h of baseline, hypoxia/carotid occlusion was induced in insult-group piglets by clamping both carotid arteries and reducing the fraction of inspired oxygen (Fio2) to 7%. The carotid clamps were released and Fio2 was returned to baseline after a randomly selected duration (10-30 min). The duration of ischemia, which is not the same as the duration of occlusion due to the possibility of collateral blood flow to the brain, was defined as the amount of time the mean arterial pressure (MAP) was less than 70% of baseline during the insult (13). CBF was measured at the point when the MAP fell to 70% of baseline to ensure the occurrence of ischemia. CMRO2, CBF, cerebral blood volume (CBV), [HHb], and oxygen extraction fraction (OEF) were measured with NIRS (10,11) three times during baseline at intervals of 20 min and then at 1, 4, 8, 12, 16, 20, and 24 h after resuscitation from hypoxia-ischemia. At 24 h, piglets were euthanized. In 9 of the 17 piglets (6 insult piglets, 3 controls), brains were harvested to look for early histologic evidence of neuronal injury using Fluoro-Jade B staining.
At all times during the experiment (excluding the insult), arterial Pco2 was maintained between 4.7-5.3 kPa by adjusting the respiratory rate, whereas arterial Po2 was maintained at 13-24 kPa by adjusting the ratio of oxygen to medical air. Blood glucose was kept between 3 and 8 mM by constant infusion of a 25% dextrose solution into a belly vein. A water-heating blanket was used to maintain a rectal temperature between 37.5 and 38.5°C. Arterial pH, MAP, and heart rate were also monitored.
Near-infrared spectroscopy.
NIRS data were collected with a continuous-wave, broadband (600-980 nm) system with a single emission fiberoptic bundle and a single detection bundle (9). The bundles were placed 3 cm apart, parasagittally on the head, proximal to the widest part of the brain.
The method used to calculate CMRO2 with NIRS, which was designed to be clinically applicable, has been discussed in detail previously (10). Briefly, measurements of CMRO2 were based on the Fick principle:

The oxygen concentration of the arteries feeding the cerebral tissue ([O2]a) was determined using a pulse oximeter from a front foot of the piglet. Measurements of [HHb] were quantified using the second-derivative technique (8) and used to indirectly determine the oxygen concentration of the vessels draining the cerebral tissue ([O2]v). The calculation of CBF and CBV required an i.v., 0.1 mg/kg bolus injection of ICG and relied on deconvolution of the subsequent arterial and tissue concentration-time curves of the tracer (9). The fraction of oxygen extracted from arterial blood into the brain, OEF, was also calculated.
Fluoro-Jade B staining.
To represent the region interrogated by the NIRS probes, coronal tissue slices were cut from the widest part of the brain and paraffin-embedded. Six sections were then cut at a thickness of 5 μm. The sections were stained with Fluoro-Jade B to assess the amount of neuronal degeneration (14). Slides were viewed with a fluorescence microscope at 20× magnification. Positive cells were then counted manually on digital images from eight cortical regions (four in each hemisphere) and two regions from the hippocampus (one in each hemisphere) by an observer blinded to treatment. The original selection of the regions was arbitrary but corresponding regions were selected for all slices and all piglets. Three replicate sections from each region for each piglet were stained and counted separately, with data averaged to yield the number of positive cells per area, per animal. At least one control section was included in each batch of staining as a standard with which to ensure the quality of the procedure.
Statistical analysis.
The statistical software package, SPSS 16.0 (SPSS, Chicago, IL), was used for all statistical analyses. Repeated-measures, two-way mixed analysis of variances (ANOVAs) were used to compare measurements between control and insult groups, with time as the within-subjects variable. The analyses were conducted for all physiologic parameters, CBF, CBV, OEF, CMRO2, and [HHb]. For parameters in which a two-way, time-by-group effect was revealed by the omnibus test, group differences at individual time points were uncovered by one-way ANOVA with Bonferroni correction to account for multiple comparisons. Linear regressions were used for all data from the insult-group piglets at each time point to uncover correlations with respect to the duration of ischemia. Statistical significance for all tests was based on a p < 0.05. All data are presented as mean ± SEM.
RESULTS
Average age and weight of the seventeen piglets were 18.9 ± 11.5 h and 1.6 ± 0.2 kg. Table 1 summarizes the results of the physiologic parameters for insult and control groups before hypoxia-ischemia and during early and late reperfusion periods (statistics, however, were done without temporal averaging). There were no significant differences between the insult group and the controls for any other physiologic parameter at any time point. Heart rate tended to increase over time in both groups; however, the trend was not significant and the values remained within a normal physiologic range. For insult-group piglets, no statistically significant correlations were found when linear regressions were conducted between each physiologic parameter and the duration of ischemia at any point of the experiment.
Figure 1 illustrates the average results of the NIRS measurements, specifically CMRO2, CBF, CBV, OEF, and [HHb] from the insult and control groups at 1, 4, 8, 12, 16, 20, and 24 h after the termination of hypoxia-ischemia. Repeated-measures ANOVA revealed a significant between-subjects interaction for the postinsult CMRO2 data [F1,15 = 23.5, p < 0.001, Power >0.99]. Further analysis uncovered that at 1, 4, 8, 12, and 24 h after the insult, average CMRO2 of the insult group was significantly lower than controls (p < 0.05). No significant time-by-group interactions were observed for CBF, CBV, or OEF postinsult. There was, however, a weak time-by-group interaction for [HHb] [F6,90 = 2.84, p < 0.05, Power >0.6]. The source of this interaction was a significantly lower [HHb] in the insult group compared with controls at 20 h after hypoxia-ischemia (p < 0.05). Furthermore, there were no significant differences between baseline measures from controls and baseline measures from insult-group piglets or postmock insult measures from controls at any time for any variable. Baseline CMRO2, CBF, CBV, and OEF were 1.9 ± 0.1 mL O2 · min−1 · 100 g−1, 46.6 ± 2.2 mL · min−1 · 100 g−1, 3.9 ± 0.1 mL · 100 g−1, and 0.31 ± 0.02, respectively.
Cerebral metabolic rate of oxygen (CMRO2), cerebral blood flow (CBF), cerebral blood volume (CBV), oxygen extraction fraction (OEF), and cerebral concentration of deoxy-Hb ([HHb]) of control and insult-group piglets after hypoxia-ischemia. Average CMRO2 (A), CBF (B), CBV (C), OEF (D), and [HHb] (E), after the termination of hypoxia-ischemia for control (dashed line; n = 5) and insult (solid line; n = 12) groups. *Control and insult groups significantly different from each other (p < 0.05). Error bars are SEM.
Table 2 presents the duration of hypoxia/occlusion and the duration of ischemia for each of the 12 insult-group piglets. Three piglets were exposed to 10 min of hypoxia/carotid occlusion, 1 piglet to 15 min, 3 piglets to 20 min, and 5 piglets to 30 min. Within the first minutes of hypoxia/occlusion, average MAP increased to 9.1 ± 0.8 kPa before crashing. The median duration of hypoxia/occlusion while MAP remained above 70% was 7 min (range, 2-15 min). CBF was calculated during this period in three piglets with an average of 32.5 ± 5.0 mL · min−1 · 100 g−1. After the initial period of hypertension, all piglets became hypotensive and MAP remained depressed for the remainder of the insult. The median duration of hypotension was 11.5 min (range, 5-28 min). CBF was successfully calculated at the onset of hypotension in 9 of the 12 insult-group piglets and the average was 7.1 ± 1.8 mL · min−1 · 100 g−1.
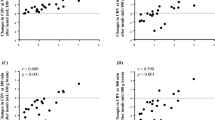
Table 3 summarizes the temporal aspects of the relationship between postinsult CMRO2 and duration of ischemia. At 1 and 4 h postinsult, the regression between CMRO2 and duration of ischemia was not statistically significant. A significant regression was reached at 8 h postinsult and the magnitude of the slope, as well as the R value of the correlations, increased sequentially at 12 and 16 h, reaching a plateau by 20 h postinsult. These correlations are illustrated in Figure 2. Figure 2A-C present individual CMRO2 values from all insult-group piglets averaged over early (1 and 4 h), mid (8 and 12 h), and late (16, 20, and 24 h) reperfusion periods, respectively, plotted against the duration of ischemia. Linear regression of all other NIRS measurements compared with the duration of ischemia yielded no statistically significant correlations at any time point except for [HHb], which demonstrated a correlation at 20 h postinsult (r = 0.80, slope = −2.7 μM per min of insult, p < 0.05).
Correlation between the cerebral metabolic rate of oxygen (CMRO2) and duration of ischemia during early, middle and late reperfusion. The CMRO2 of insult-group piglets (n = 12) averaged over 1 and 4 h postinsult (A), 8 and 12 h postinsult (B), and 16, 20, and 24 h postinsult (C), plotted against duration of ischemia. Solid lines indicate fits of regression (for A: slope = −0.005 mL O2 · min−1 · 100 g−1 · min of insult−1, r = 0.22, NS, for B: slope = −0.019 mL O2 · min−1 · 100 g−1 · min of insult−1, r = 0.78, p < 0.05 and for C: slope = −0.027 mL O2 · min−1 · 100 g−1 · min of insult−1, r = 0.92, p < 0.05).
Figure 3 presents the average number of Fluoro-Jade B-positive cells in cortical and hippocampal regions of six insult-group piglet brains plotted against duration of ischemia. Examples of Fluoro-Jade B stains from the cerebral cortex and hippocampus of one severely injured animal and one control animal are also presented. In both the cortex and the hippocampus, the number of Fluoro-Jade B-positive cells demonstrated statistically significant correlations with insult duration (r = 0.85, p < 0.05 and r = 0.94, p < 0.05, respectively). The average number of Fluoro-Jade B-positive cells in the cerebral cortex and the hippocampus from control animals was 3.8 ± 0.5 and 2.9 ± 0.4, respectively.
Histology. Figure (A) presents the average number of Fluoro Jade B-positive cells in the cortex of six piglets subjected to varying durations of hypoxia-ischemia plotted against duration of ischemia, coupled with corresponding images from a control animal (B) and a severe animal (C). Figure (D) presents the average number of Fluoro Jade B-positive cells in the hippocampus plotted against duration of ischemia, coupled with corresponding images from a control animal (E) and a severe animal (F). The scale bars in each photomicrograph indicate 100 μm.
DISCUSSION
The delay between cerebral hypoxia-ischemia and brain injury in the newborn has led to the idea that diagnosis and treatment of hypoxia-ischemia during early reperfusion could improve long-term neurologic outcome. In general, current clinical methods of diagnosing the occurrence of a hypoxic-ischemic event have proven to suffer from poor specificity (15); consequently, there remains a demand for novel diagnostic schemes. Reduced cerebral energy metabolism has shown promise as an early indicator of hypoxia-ischemia (4,5); however, traditional methods for measuring cerebral energy metabolism are poorly suited for neonates. Recently, a robust NIRS method for safely and accurately measuring cerebral oxidative metabolism was developed in our laboratory and validated over a range of metabolic states and after hypoxic-ischemic injury in the piglet (10–12). The purpose of the present study was to test the hypothesis that an early measure of CMRO2 after hypoxia-ischemia—acquired with the described NIRS technique—can be used as an indicator of the severity of insult incurred.
Similar approaches for measuring CMRO2 have been published that have used oxy-Hb as an endogenous blood flow indicator after a rapid change in Fio2 (16). The main advantage with the present technique is the increased precision of the CBF measurements gained by using ICG as a tracer (17). The precision was further increased (to less than 10%) by using a deconvolution method designed to account for variable arrival times to calculate CBF, which allowed data outside of the first pass of the bolus to be included, and also allowed CBV to be calculated (9). Although ICG is approved for use in newborns in North America, there is some risk: the incidence of systemic allergic reactions to the dye has been reported as approximately 1:250,000 in adults and newborns (18). Recently, however, a hypoallergenic form of ICG, infracyanine (SERB Laboratories, Paris, France), has been developed.
The present study demonstrated that the CMRO2 response after hypoxia-ischemia could be used to distinguish hypoxic-ischemic piglets (over a range of severities) from controls. In the early stages after resuscitation, there were no significant correlations when the CMRO2 of insult-group piglets was compared with the duration of ischemia; however, as a group, the average CMRO2 of the insult piglets was significantly lower than that of controls. Subsequently, the CMRO2 of piglets that were subjected to shorter durations of ischemia was found to gradually return toward control levels, whereas the CMRO2 of piglets that were subjected to longer durations of ischemia remained depressed. This was evident by the emergence of a significant correlation between postinsult CMRO2 and duration of ischemia (Table 3) and the gradual increase of mean insult-group CMRO2 (Fig. 1).
Of the parameters used to calculate CMRO2—namely CBF, CBV and [HHb], only [HHb] revealed any statistically significant group differences after hypoxia-ischemia. Specifically, average [HHb] was reduced in the insult group compared with controls at 20 h after the insult. Also at the 20 h mark, the [HHb] of the insult-group piglets had a statistically significant correlation with the duration of ischemia (p < 0.05). However, the fact that there was only a significant difference at one time point—in addition to the results of Peeters-Scholte et al., (19) who found no change in [HHb] from baseline out to 24 h after severe hypoxia-ischemia in piglets—refutes the idea of using [HHb] in a prognostic capacity. In a previous study from our group, CMRO2 was monitored for 6 h after resuscitation in piglets subjected to 30 min of hypoxia-ischemia (12); in support of the results from the present study, even though hypoxia-ischemia caused a significant and persistent 25% reduction in CMRO2, there were no group effects with the parameters used to determine CMRO2. The insensitivity of these parameters to hypoxia-ischemia highlights the importance of measuring CMRO2 for diagnostic purposes.
CBF tended to be lower in insult-group piglets than controls in the first 4 h after resuscitation although the between-groups effect was not statistically significant. Reduced CBF, combined with reduced CMRO2, has been observed after hypoxia-ischemia in the neonatal lamb (5). Under normal conditions, CBF and CMRO2 are tightly coupled and if CMRO2 was observed to drop by approximately 30%, as seen in the present study, a similar drop would be expected in CBF. The apparent lack in sensitivity of early CBF measurements to insult duration and the complete inability to use CBF to discern between groups late in the experiment is possibly linked to a hypoxia-ischemia-induced loss of autoregulation, and subsequent uncoupling from metabolism (20).
Despite clamping of both carotid arteries during the insult, some piglets may be able to maintain adequate CBF by increasing flow to collateral vertebral arteries (21). The CBF measurements in the early insult window acquired from three piglets confirmed that relatively normal levels of CBF could be maintained despite bicarotid occlusion due to retaliatory hypertension. It is known that hypoxia alone is not nearly as severe as the combination of both hypoxia and ischemia (22); therefore, to ensure an accurate representation of the duration of ischemia for each piglet, the starting time of the insult was defined as the point when MAP dropped below 70% of baseline (13). This threshold is the lower limit of autoregulation, beyond which, CBF becomes pressure-dependent and, therefore, any collateral flow would drop. The decision to use the 70% MAP threshold is further reinforced by the results of CBF measurements made at the onset of hypotension, which demonstrated that blood flow had reached ischemic levels (<15 mL · min−1 · 100 g−1). Furthermore, in a previous study we found that the average CBF during hypoxia/occlusion was 36 ± 6 mL · min−1 · 100 g−1 when MAP was greater than 70% of baseline and 8 ± 3 mL · min−1 · 100 g−1 when MAP was less than 70% (12). The duration of hypoxia without ischemia is not considered to be a large contributor to brain injury in this study since the maximum duration of hypoxia alone was only 15 min (Table 2).
The amount of Fluro-Jade B positive staining also correlated with the defined duration of ischemia however the n size was too small for the data to be conclusive. Fluoro-Jade B was chosen because it provides an early indication of neuronal degeneration with a high signal-to-noise ratio (14) and the purpose of its use was to assess the extent of brain damage resulting from a range of ischemic durations. The results suggested that durations of ischemia of 15 min or greater resulted in considerable injury to the cortex and hippocampus whereas shorter durations of ischemia (two piglets exposed to 5 min of hypoxia-ischemia) resulted in a quantity of staining similar to controls brains, which showed no signs of pathology. Correspondingly, the CMRO2 of these two piglets with shorter durations of ischemia returned to within 1 SD of control levels by 8 h of reperfusion. It is possible, however, that the damage in these piglets may have been delayed so that it was not detected at 24 h. Studies are ongoing to address these limitations by conducting experiments with a 72 h postinsult recovery period and with a focus on mild to moderate injuries, which are arguably more difficult to detect clinically.
The importance of early prognosis warrants further discussion regarding the effects of hypoxia-ischemia on CMRO2 in the first hours after reperfusion. The results of the present study suggest that CMRO2 is very sensitive to hypoxia-ischemia during this window, regardless of the duration of ischemia since the average CMRO2 of all insult-group piglets was 33 ± 3% lower than that of control piglets; however, there was no significant correlation between CMRO2 of insult-group piglets and duration of ischemia until 8 h postinsult. Hypoxia-ischemia has been shown to trigger long-term depression of cerebral metabolism in both adult (3,23) and neonatal (4,5) animal models. Interestingly, this depression has been observed despite a restitution of high-energy metabolites in awake rats that showed exploratory behavior and recovery of motor functions (23). Potential causes of the postischemic cerebral depression observed in this study include the prolonged effect of a release of adenosine, which occurs during hypoxia and ischemia and acts by inhibiting synaptic activity (24); mitochondrial dysfunction, caused by either ischemic damage or inhibition by the postischemic release of nitric oxide (25); or perhaps an increased sensitivity to the anesthetic agent, isoflurane, which could have caused an increase in mean alveolar concentration and possibly a reduction in synaptic activity that would have reduced metabolic demand (26).
Because of the limitations of this study, it is not possible to comment conclusively on the exact mechanism for the postischemic cerebral depression. If the effect was related to the use of isoflurane, it is feasible that the correlation between CMRO2 and the duration of ischemia observed at 8 h postinsult emerged as the increased sensitivity to isoflurane abated. In general, infants suspected of sustaining a hypoxic-ischemic insult are not anesthetized; therefore, it is possible that the sensitivity of CMRO2 to insult severity will occur much earlier after resuscitation clinically, creating a potential for prognosis of insult severity with NIRS during early reperfusion. Furthermore, the greater energy demand of the infant compared with the anesthetized piglet could increase the sensitivity of the NIRS measurement by widening the difference between CMRO2 of healthy and injured patients. In piglets, awake levels of CMRO2 are roughly 30% higher than levels under isoflurane in piglets (11). Studies are continuing in our laboratory to explore the relationship between CMRO2 and insult severity during early reperfusion using a combination of fentanyl and nitrous oxide for anesthesia. This combination has been demonstrated to have little effect on cerebral metabolism (27).
To the best of the authors' knowledge, this is the first study to find a relationship between CMRO2 and hypoxic-ischemic insult severity. Furthermore, CMRO2 was measured using a NIRS apparatus designed for rapid and repeated use at the bedside, strengthening the proposal that NIRS may be an effective tool for prognosis and treatment planning of hypoxic-ischemic encephalopathy in critically ill newborns.
Abbreviations
- CBF:
-
cerebral blood flow
- CBV:
-
cerebral blood volume
- CMRO2:
-
cerebral metabolic rate of oxygen
- Fio2:
-
fraction of inspired oxygen
- HHb:
-
deoxy-hemoglobin
- ICG:
-
indocyanine green
- NIRS:
-
near-infrared spectroscopy
- MAP:
-
mean arterial pressure
- OEF:
-
oxygen extraction fraction
References
Sunshine P 2002 Hypoxic-ischemic encephalopathy: pathophysiology and implications for therapy. Przegl Lek 59: 6–9
du Plessis AJ, Volpe JJ 2002 Perinatal brain injury in the preterm and term newborn. Curr Opin Neurol 15: 151–157
Sims NR, Zaidan E 1995 Biochemical changes associated with selective neuronal death following short-term cerebral ischaemia. Int J Biochem Cell Biol 27: 531–550
Thorngren-Jerneck K, Ley D, Hellstrom-Westas L, Hernandez-Andrade E, Lingman G, Ohlsson T, Oskarsson G, Pesonen E, Sandell A, Strand SE, Werner O, Marsal K 2001 Reduced postnatal cerebral glucose metabolism measured by PET after asphyxia in near term fetal lambs. J Neurosci Res 66: 844–850
Rosenberg AA 1986 Cerebral blood flow and O2 metabolism after asphyxia in neonatal lambs. Pediatr Res 20: 778–782
Hanrahan JD, Cox IJ, Azzopardi D, Cowan FM, Sargentoni J, Bell JD, Bryant DJ, Edwards AD 1999 Relation between proton magnetic resonance spectroscopy within 18 hours of birth asphyxia and neurodevelopment at 1 year of age. Dev Med Child Neurol 41: 76–82
Martin E, Buchli R, Ritter S, Schmid R, Largo RH, Boltshauser E, Fanconi S, Duc G, Rumpel H 1996 Diagnostic and prognostic value of cerebral 31P magnetic resonance spectroscopy in neonates with perinatal asphyxia. Pediatr Res 40: 749–758
Matcher SJ, Cope M, Delpy DT 1994 Use of the water absorption spectrum to quantify tissue chromophore concentration changes in near-infrared spectroscopy. Phys Med Biol 39: 177–196
Brown DW, Picot PA, Naeini JG, Springett R, Delpy DT, Lee TY 2002 Quantitative near infrared spectroscopy measurement of cerebral hemodynamics in newborn piglets. Pediatr Res 51: 564–570
Brown DW, Hadway J, Lee TY 2003 Near-infrared spectroscopy measurement of oxygen extraction fraction and cerebral metabolic rate of oxygen in newborn piglets. Pediatr Res 54: 861–867
Tichauer KM, Hadway JA, Lee TY, St Lawrence K 2006 Measurement of cerebral oxidative metabolism with near-infrared spectroscopy: a validation study. J Cereb Blood Flow Metab 26: 722–730
Tichauer KM, Brown DW, Hadway J, Lee TY, St Lawrence K 2006 Near-infrared spectroscopy measurements of cerebral blood flow and oxygen consumption following hypoxia-ischemia in newborn piglets. J Appl Physiol 100: 850–857
Foster KA, Colditz PB, Lingwood BE, Burke C, Dunster KR, Roberts MS 2001 An improved survival model of hypoxia/ischaemia in the piglet suitable for neuroprotection studies. Brain Res 919: 122–131
Schmued LC, Hopkins KJ 2000 Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res 874: 123–130
Volpe JJ 2001 Neurology of the Newborn. Philadelphia: W.B. Saunders Company
Yoxall CW, Weindling AM 1998 Measurement of cerebral oxygen consumption in the human neonate using near infrared spectroscopy: cerebral oxygen consumption increases with advancing gestational age. Pediatr Res 44: 283–290
Patel J, Marks K, Roberts I, Azzopardi D, Edwards AD 1998 Measurement of cerebral blood flow in newborn infants using near infrared spectroscopy with indocyanine green. Pediatr Res 43: 34–39
Garski TR, Staller BJ, Hepner G, Banka VS, Finney RA Jr 1978 Adverse reactions after administration of indocyanine green. JAMA 240: 635
Peeters-Scholte C, van den Tweel E, Ioroi T, Post I, Braun K, Veldhuis W, Nicolay K, Groenendaal F, van Bel F 2002 Pharmacological interventions in the newborn piglet in the first 24 h after hypoxia-ischemia. A hemodynamic and electrophysiological perspective. Exp Brain Res 147: 200–208
Lou HC, Lassen NA, Tweed WA, Johnson G, Jones M, Palahniuk RJ 1979 Pressure passive cerebral blood flow and breakdown of the blood-brain barrier in experimental fetal asphyxia. Acta Paediatr Scand 68: 57–63
Munkeby BH, Lyng K, Froen JF, Winther-Larssen EH, Rosland JH, Smith HJ, Saugstad OD, Bjornerud A 2004 Morphological and hemodynamic magnetic resonance assessment of early neonatal brain injury in a piglet model. J Magn Reson Imaging 20: 8–15
Rice JE III, Vannucci RC, Brierley JB 1981 The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 9: 131–141
Kozuka M, Smith ML, Siesjo BK 1989 Preischemic hyperglycemia enhances postischemic depression of cerebral metabolic rate. J Cereb Blood Flow Metab 9: 478–490
Martin ED, Fernandez M, Perea G, Pascual O, Haydon PG, Araque A, Cena V 2007 Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission. Glia 55: 36–45
Hagberg H 2004 Mitochondrial impairment in the developing brain after hypoxia-ischemia. J Bioenerg Biomembr 36: 369–373
Rampil IJ, Weiskopf RB, Brown JG, Eger EI II, Johnson BH, Holmes MA, Donegan JH 1988 I653 and isoflurane produce similar dose-related changes in the electroencephalogram of pigs. Anesthesiology 69: 298–302
Yaster M, Koehler RC, Traystman RJ 1994 Interaction of fentanyl and nitrous oxide on peripheral and cerebral hemodynamics in newborn lambs. Anesthesiology 80: 364–371
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by Canadian Institutes of Health Research (CIHR).
Rights and permissions
About this article
Cite this article
Tichauer, K., Wong, D., Hadway, J. et al. Assessing the Severity of Perinatal Hypoxia-Ischemia in Piglets Using Near-Infrared Spectroscopy to Measure the Cerebral Metabolic Rate of Oxygen. Pediatr Res 65, 301–306 (2009). https://doi.org/10.1203/PDR.0b013e318194faa6
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e318194faa6
This article is cited by
-
RETRACTED ARTICLE: Metformin Attenuates Cognitive Impairments in Hypoxia–Ischemia Neonatal Rats via Improving Remyelination
Cellular and Molecular Neurobiology (2017)