Abstract
High-frequency oscillatory ventilation (HFOV) may improve pulmonary outcome in very preterm infants, but the effects on the brain are largely unknown. We hypothesized that early prolonged HFOV compared with low volume positive pressure ventilation (LV-PPV) would not increase the risk of delayed brain growth or injury in a primate model of neonatal chronic lung disease. Baboons were delivered at 127 ± 1 d gestation (dg; term ∼185 dg), ventilated for 22–29 d with either LV-PPV (n = 6) or HFOV (n = 5). Gestational controls were delivered at 153 dg (n = 4). Brains were assessed using quantitative histology. Body, brain, and cerebellar weights were lower in both groups of prematurely delivered animals compared with controls; the brain to body weight ratio was higher in HFOV compared with LV-PPV, and the surface folding index was lower in the LV-PPV compared with controls. In both ventilated groups compared with controls, there was an increase in astrocytes and microglia and a decrease in oligodendrocytes (p < 0.05) in the forebrain and a decrease in cerebellar granule cell proliferation (p < 0.01); there was no difference between ventilated groups. LV-PPV and HFOV ventilation in prematurely delivered animals is associated with decreased brain growth and an increase in subtle neuropathologies; HFOV may minimize adverse effects on brain growth.
Similar content being viewed by others
Main
Advances in prenatal and neonatal care, including respiratory support, have significantly improved survival rates for prematurely delivered infants in recent years. These infants, however, still have a higher incidence of poor neurodevelopmental outcome than full-term infants with approximately 10% at risk of developing cerebral palsy and 10–20% likely to have some form of developmental delay or sensory or motor impairment (1,2). Thus, it is critical to understand the effects of postnatal intervention on the developing brain and, in particular, to determine whether specific modes of respiratory support and other postnatal treatments influence the nature and severity of cerebral injury.
High-frequency oscillatory ventilation (HFOV) is used in the neonatal setting as both primary and rescue therapy, because it is believed to cause less trauma to the immature lungs compared with other forms of mechanical ventilation. In models of neonatal lung injury, HFOV has been shown to improve pulmonary outcome (3,4). Recent meta-analyses in human studies have cast some doubt on whether there is a better prognosis for chronic lung disease (CLD) after HFOV compared with an optimized protocol of conventional mechanical ventilation (5,6). The effects on the brain are also controversial with some studies suggesting an increased risk of both intracranial hemorrhage and periventricular leucomalacia (7,8) and others reporting that there is no difference in neonatal associated morbidity, including hemorrhage (9). Long-term neurodevelopmental (10) and neuromotor (11) outcomes do not seem to be worse in infants ventilated with HFOV compared with conventional ventilation.
Neuropathological analysis of the effects that specific respiratory regimens have on the human preterm brain are likely to be complicated by the processes which contributed to the infant's death. An animal model with brain and cardiopulmonary development comparable with the human preterm neonate, managed in a neonatal intensive care environment closely mimicking the current approach for humans, may provide a better understanding of the effects of various interventional support strategies. We have established that the prematurely delivered baboon at 125 d of gestation (dg) has similar brain (12) and cardiorespiratory (13) development to the very preterm human infant at about 26–28 wk of gestation and is, thus, a highly appropriate model for such investigations. Our aim was to deliver baboon neonates at 125 dg and to study the brain growth and the pattern of cerebral injury in this model of neonatal CLD following early, prolonged HFOV compared with low tidal volume positive pressure ventilation (LV-PPV). We hypothesized that early sustained HFOV compared with LV-PPV would not increase the risk of brain injury and/or altered brain development and growth associated with prematurity.
MATERIALS AND METHODS
Studies were performed at the Southwest Foundation for Biomedical Research, San Antonio, TX. All animal husbandry, handling, and procedures were approved to conform to American Association for Accreditation of Laboratory Animal Care guidelines.
Delivery and instrumentation.
Pregnant baboon dams (Papio papio) were treated with 6 mg of intramuscular betamethasone 48 and 24 h before elective hysterotomy under general anesthesia. Study animals were delivered at 127 ± 1 dg (term ∼185 dg). At birth, animals were weighed, sedated, intubated, and treated with 4 mL/kg bolus of exogenous surfactant (Survanta, courtesy Ross Laboratories, Columbus, OH) through the endotracheal tube. For gestational control brains, additional pregnant baboon dams were treated with 6 mg of intramuscular betamethasone at 123 and 124 dg and animals (n = 4) delivered by elective hysterotomy at 153 dg; animals were killed immediately.
Respiratory management.
Management of ventilated animals has been described in detail previously (3). At 5 min of age, based on prior antenatal assignment, the animals were converted to HFOV or maintained on low tidal volume (4–6 mL/kg) PPV. Similar goals were set and achieved for oxygenation (55–70 torr) and ventilation (45–55 torr). Surgical ductal ligation was performed on three animals in the HFOV group and one animal in the LV-PPV group. Three other animals in the LV-PPV group were treated with indomethacin for patent ductus arteriosus. More detailed methods related to management of nutrition, ventilation, blood pressure, and infection can be found in a previous publication (3).
Tissue collection and processing.
Brains were weighed, immersed in 4% paraformaldehyde in 0.1 M phosphate buffer, and coronal blocks from the right forebrain (at 5 mm intervals) and a mid-sagittal block from the cerebellar vermis of each brain were processed to paraffin. Ten 8-μm sections were cut from the rostral surface of each forebrain block (10–12 per animal) and in the sagittal plane for the cerebellum.
Morphologic analysis.
Analyses were performed on sections from each block for all brains of LV-PPV, HFOV, and gestational control animals; measurements were made on coded slides. Areas and widths were assessed using a digitizing program (Sigma Chemical Co. Scan Pro v4, SPSS Science, Chicago, IL); optical density estimates and counts performed using an image analysis system (Image Pro Plus v4.1, Media Cybernetics, MD). Means were calculated for each animal, and then a group mean was determined.
Sections were stained with hematoxylin and eosin (H&E) and assessed qualitatively for gross morphologic changes, including lesions and the presence of hemorrhages (scored: present, 1; absent, 0).
Immunohistochemistry for rabbit anti-rat calbindin (1:500, Swant, Bellinzona, Switzerland) was used to identify Purkinje cells; rabbit anti-cow glial fibrillary acid protein (GFAP; 1:500, Sigma Chemical Co., St. Louis, MO) to identify astrocytes; mouse anti-human Ki-67 clone MIB-1 (1:100; DakoCytomation, Denmark) to identify mitotic cells; rat anti-bovine myelin basic protein (MBP, 1:100; Chemicon, CA) to assess the extent of myelination; rabbit anti-ionized calcium-binding adapter molecule 1 (Iba1, 1:1500, Wako Chemicals, Osaka, Japan) to identify microglia or macrophages; and rabbit anti-human p27 (Kip1) cyclin-dependent kinase inhibitor (1:1000, Millipore, Billerica, MA) to identify postmitotic cells, as previously described (14). Control and experimental materials were stained simultaneously to avoid procedural variation. Control experiments were performed omitting the primary antibodies whereupon staining failed to occur.
Quantitative analysis.
The following measurements were made in the forebrain: volumes of the white and gray matter (GM) components (12); surface folding index (SFI; 12); areal densities of astrocytes in the neocortex, white matter (WM) and hippocampus (15); somal areas and areal densities of oligodendrocytes in the WM (15); and areal densities of microglia in the neocortex and WM. The following measurements were made in the cerebellum: areas of cerebellar layers (14); Purkinje cell (14) and granule cell somal areas; Ki67-immunoreactivity (IR) cell counts in the external granule layer (EGL) and WM (14); areal densities of oligodendrocytes and microglia in the deep WM (14); and GFAP-IR OD in the deep WM (14) (see supplementary material, Supplemental Digital Content 1, http://links.lww.com/PDR/A50).
Semiquantitative analysis.
MBP-IR was scored against control standards for the forebrain (15) and cerebellum (14).
GFAP-IR radial glial fibers in the forebrain were scored on a scale of 0–3 (15).
Physiologic data.
Physiologic data, including arterial blood gases [PaO2, Po2, PaCO2, Pco2, pH, fraction of inspired oxygen (FiO2)], mean arterial blood pressure (MAP), and heart rate, were monitored throughout the experimental period, and mean values were calculated. The “interval flux” of physiologic parameters was calculated as a surrogate measure of instability as described previously (14). For each animal, we then: 1) identified the maximum flux and 2) calculated the mean of the interval fluxes over the experimental period.
Statistical analysis.
Linear regression analysis was carried out to determine whether there was a correlation between: a) physiologic variables and quantitative parameters; b) quantitative parameters and volumetric measurements. Significance of differences between ventilated and control groups was tested using a one-way ANOVA with posthoc analysis (Tukey's test) for histologic parameters; t tests were used to compare between ventilated groups for physiologic parameters; a probability of p < 0.05 was considered to be significant. Results are expressed as mean ± SEM (weights and areas) and mean of means ± SEM (histologic parameters).
RESULTS
Birth weight was lower (p < 0.03) in HFOV (353 ± 18 g) compared with LV-PPV (406 ± 11 g) animals. The gestational age at birth (LV-PPV, 126 ± 1 dg versus HFOV, 127 ± 1 dg), age at postmortem (LV-PPV, 26 ± 1 d versus HFOV, 27 ± 1 d), and male/female ratios (LV-PPV, 4/2 versus HFOV, 2/3) were not different between groups.
Growth and development.
At necropsy body, brain and cerebellar weights were lower in both groups of prematurely delivered animals compared with gestational controls (p < 0.01; Table 1). The body weight was lower (p < 0.05) and the brain to body weight ratio was higher (p < 0.01) in HFOV compared with LV-PPV. The cerebellar or body weight ratio was lower in the LV-PPV compared with control (p < 0.05) and HFOV (p < 0.01) animals (Table 1). The cerebellar or brain weight was not different between groups (p > 0.05). The failure of HFOV animals to gain body weight during the 28 d of the study might be due to increased caloric use during periods of extubation.
Forebrain
Compared with gestational controls, the total, neocortical, WM, and deep GM volumes were reduced (p < 0.001) in both prematurely delivered groups (Table 2). Although ventricular volume in absolute terms was not different (p > 0.05) from controls in either group, when expressed as a percentage of the total hemispheric volume, it was increased in both LV-PPV (p < 0.01) and HFOV (p < 0.001) animals. The SFI was reduced in the LV-PPV (p < 0.05) but not in HFOV compared with controls.
Neuropathology.
No focal necrotic lesions or hemorrhages were observed in any animal. Examination of Ki-67-IR- sections showed that cell proliferation was minimal in the subventricular and subgranular zones at 153 dg in all animals; no differences were observed between groups.
Areal density of astrocytes.
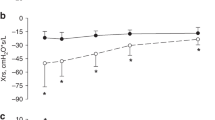
There was an increase in density in the deep (p < 0.001) and subcortical WM (p < 0.01; Fig. 1A–C), neocortex (p < 0.05), and the stratum radiatum of the hippocampus (p < 0.01) in the LV-PPV and HFOV animals compared with controls but no difference between ventilated groups (Table 3).
GFAP-IR astrocyte areal density was increased in the deep WM in the LV-PPV (B) and HFOV (C) animals compared with controls (A); arrows indicate astrocytes. D–F, The areal density of MBP-IR oligodendrocytes in cerebral white matter was reduced in LV-PPV (E) compared with HFOV (F) and controls animals (D), arrows indicate MBP-IR cell bodies. G–I, The areal density of Iba-IR macrophages or microglia was increased in the subcortical white matter in LV-PPV (H) and HFOV (I) compared with control (G) animals. A–C = 60 μm; D–F = 75 μm; G–I = 40 μm.
Areal density of MBP-IR oligodendrocytes.
There was a reduction (p < 0.05) in density in the deep (Fig. 1D–F) and subcortical WM in the LV-PPV and HFOV animals compared with controls (Table 3). There was a reduction in the somal areas of MBP-IR oligodendrocytes in the deep (p < 0.05; control, 84.3 ± 2.6 μm2; LV-PPV, 70.8 ± 3.3 μm2; HFOV, 66.5 ± 2.8 μm2) but not subcortical WM (p > 0.05) in ventilated animals compared with controls. There was no difference between ventilated groups in any of these parameters.
Areal density of microglia.
Ramified Iba1-IR cells were increased in subcortical WM (Fig. 1G–I) and neocortex in the LV-PPV and HFOV groups compared with controls (p < 0.05); there was no difference between ventilated groups (Table 3). Activated microglia (round morphology and attenuated processes) were observed infrequently with no greater incidence in prematurely delivered groups compared with gestational controls.
Forebrain semiquantitative assessment.
GFAP-IR radial glia were rarely observed in control brains at 153 dg but evident at the ventricular surface projecting into the deep and occasionally the subcortical WM in the LV-PPV (p < 0.05) and HFOV (p < 0.01) animals (Table 3).
Cerebellum
There was no evidence of hemorrhages or overt damage.
Quantitative morphologic assessment.
The cross-sectional area of the vermis was reduced in the LV-PPV (p < 0.01) and HFOV (p < 0.05) animals compared with controls, as was the volume of the inner granule layer (IGL) (p < 0.001), and the widths of the EGL (p < 0.001; Fig. 2A–C) and the molecular layer (ML) (p < 0.01; Fig. 2A–C; Table 4). The WM volume was not different between ventilated groups (p > 0.05).
A–C, The width of the cerebellar EGL and ML are reduced in LV-PPV (B, E) and HFOV (C, F) compared with control (A, D) animals (lobule 1 shown). Somal areas of IGL cells were reduced in LV-PPV (B inset) and HFOV (C inset) compared with control (A inset) animals. D–F, The proportion of Ki67-IR/non-IR cells in the EGL was increased in LV-PPV (E) compared with HFOV (F) and control (C). A–C = 120 μm; A–C inset = 17 μm; D–F = 20 μm.
For the following parameters, data from lobules 1 and 8 have been pooled, because there was no difference between lobules.
The number of Ki-67-IR cells in the EGL was reduced in the LV-PPV and HFOV animals compared with controls (p < 0.001; Table 5; Fig. 2D–F). In control and HFOV animals, approximately 50% of cells in the EGL were Ki-67-IR; in LV-PPV animals, this was higher (64%; p < 0.05; Table 5), suggesting abnormalities in the cell cycle. In all animals, EGL cells not Ki-67-IR were P27-IR (data not shown). There was a decrease in Ki67-IR Bergmann glial cells in ventilated animals compared with controls (p < 0.001). Ki67-IR cells were seen throughout the deep WM; there was no difference (p > 0.05) between groups.
Somal areas were reduced (p < 0.001), and the areal density of Purkinje cells increased in lobule 8 (p < 0.05) in ventilated animals compared with controls (Table 5).
Areal density of microglia.
Ramified Iba-IR cells were observed throughout the cerebella in all groups; the density was higher (p < 0.05) in the ML in ventilated animals compared with controls; there was no difference between groups in the IGL (Table 5; p > 0.05).
IGL somal areas were reduced (Fig. 2A–C inset) in LV-PPV (p < 0.01) and HFOV (p < 0.05) animals compared with controls (Table 5).
Areal density of MBP-IR oligodendrocytes and OD of GFAP-IR in the WM.
There was no difference (p > 0.05) between groups in either parameter (Table 5).
Physiologic responses.
There was no difference (p > 0.05) in the mean interval flux of pH, PAo2, PaCO2, FiO2, MAP, or heart rate between LV-PPV and HFOV animals during the 28-d study period (Table S1, Supplemental Digital Content 1, http://links.lww.com/PDR/A50).
Relationship between physiologic variables and morphologic parameters.
There were negative correlations between: the mean interval flux in FiO2 and both the density of deep WM oligodendrocytes (r2 = 0.42; p < 0.03) and forebrain WM volume (r2 = 0.45; p < 0.02); density of astrocytes and density of oligodendrocytes in the deep WM (r2 = 0.47, p < 0.005); and forebrain WM volume and density of astrocytes (r2 = 0.79, p < 0.0001). There were positive correlations between the mean interval flux in FiO2 and the density of hippocampal astrocytes (r2 = 0.37; p < 0.05) and between the density of astrocytes and density of microglia in both the deep (r2 = 0.50; p < 0.03) and subcortical (r2 = 0.54; p < 0.002) WM; there were no correlations between any physiologic parameters and structural alterations in the cerebellum.
DISCUSSION
We have demonstrated that long-term ventilation of the prematurely delivered baboon neonate with both LV-PPV and HFOV is associated with reduced brain growth and an increase in subtle brain injury compared with gestational controls. Animals ventilated with HFOV were not at a greater risk of injury or reduced brain growth than those ventilated with LV-PPV; neither regimen was associated with hemorrhage or overt GM or WM damage. Our findings, therefore, concur with human studies, which have shown that HFOV is not associated with an increased risk of neonatal morbidity, including intracranial hemorrhage (1,2). In translating our findings to the human neonate, we acknowledge that they are based on small animal numbers, and that preterm baboons were delivered without any preexisting complications, such as infection, hypoxemia, or growth restriction.
Brain growth and development.
Premature delivery and prolonged ventilation with either HFOV or LV-PPV was associated with a reduction in brain weights and volumes compared with controls; however, the brain to body weight ratio was higher in animals ventilated with HFOV than with LV-PPV. The SFI, a measure of cortical gyrification was reduced in LV-PPV but not in HFOV animals compared with controls. Gyrification of the cerebral hemispheres is believed to reflect development of cerebral and subcortical connectivity (16), hence connectivity might be reduced or delayed in the LV-PPV animals. Taken together, these data suggest that HFOV ventilation might minimize the adverse effects of very preterm birth on brain growth.
Forebrain neuropathology.
We observed subtle neuropathologic alterations in GM and WM in both groups of prematurely delivered animals; however, there was no difference between groups in any of the quantitative parameters. In both groups, we identified mild ventriculomegaly, which could result from cell loss or a lack of growth of the neuropile; ventriculomegaly is associated with an increased risk of mild to moderate learning difficulties (17). Astrogliosis was observed throughout the forebrain in both prematurely delivered groups. Reactive astrocytes produce IGF-1 and FGF (18) to support neuronal survival, but they also produce cytokines (19) and reactive oxygen species, which could exacerbate any underlying injury through oxidative and inflammatory pathways. Microgliosis was observed in the cortex and subcortical WM; these microglia were predominantly in a ramified state. Microglia might be playing either a protective role by releasing growth factors or, when activated, a harmful role through the release of cytokines (20), which will exacerbate any neuronal injury.
Radial glial fibers, which were not present in control animals at 153 dg, were evident in the forebrain of both groups of prematurely delivered animals. It is possible that the normal maturation of radial glia into astrocytes has been inhibited by cerebral hypoxia leading to their prolonged presence in the developing brain (18). Alternatively, hypoxia might have caused astrocytes to revert to radial glia (18) in an attempt to repair damage as radial glia are neuronal precursors. Although every effort is made in the NICU to keep blood oxygen saturation at optimal levels, immaturity of respiratory control means that periods of mild hypoxemia might occur.
The reduction in both somal size and density of MBP-IR myelinating oligodendrocytes in the WM of LV-PPV and HFOV animals compared with controls suggests a significant effect on myelination with consequences for the normal functioning of axonal pathways. No cystic infarction in the WM was noted in any animal. The diffuse pattern and more subtle nature of injury in the preterm baboon brain are consistent with that which is reported in recent neuroimaging studies in the preterm infant (21).
Cerebellar morphologic alterations.
We observed microstructural alterations within the vermis in both groups of prematurely delivered animals. Cerebellar weights and morphologic parameters were comparable with those seen between 125 and 140 dg (14), suggesting developmental delay. We posit that specific alterations in developmental events are also involved, particularly reduced proliferation of granule cells; the EGL was significantly thinner than it would be at any stage of normal development (14). This effect on neurogenesis was the most profound that we have observed with any of the postnatal intervention strategies investigated in the preterm baboon (12,14,15,22). The reduction in Bergmann glial cell density in ventilated animals could affect granule cell migration and influence Purkinje cell dendritic alignment (23). The reduction in Purkinje cell somal size is likely to be associated with reduced dendritic growth (24) and account for the thinner ML and increased Purkinje cell density. These alterations could have a long lasting effect on the complex pattern of cerebellar connectivity and hence function.
Microgliosis in the ML suggests an underlying inflammatory response with possible consequences similar to those mentioned earlier for the forebrain. Unlike the forebrain, myelination did not seem to be affected in the cerebellum.
Mechanisms underlying vulnerability of the preterm brain.
The mechanisms underlying reduced growth and injury in both HFOV and LV-PPV groups compared with gestational controls are likely to be multifactorial and could include altered nutritional status, fluctuations in blood gases, length of time intubated, and subsequent activation of inflammatory cascades. The finding that HFOV seemed to minimize adverse outcomes on the brain growth might relate to more favorable oxygenation; HFOV animals have better lung function with sustained improvement in pulmonary mechanics compared with animals managed with LV-PPV (3). In all ventilated animals, higher interval fluxes in FiO2 were associated with increased astrocyte densities in the hippocampus and reduced oligodendrocyte densities and forebrain WM volume, suggesting that greater variation in oxygen delivery seems to result in a greater level of brain injury.
In both LV-PPV and HFOV groups, cerebellar growth was more markedly affected than forebrain growth possibly as a consequence of the rapid growth of this structure during the premature period (14,25) and hence its enhanced vulnerability.
Long-term ventilation with HFOV or LV-PPV appears to result in poorer outcome for the brain than nCPAP.
HFOV and LV-PPV have poorer outcomes for the brain growth and more injury and structural alterations than previously observed after 28 d of a more passive ventilatory regimen, nasal continuous positive airway pressure (nCPAP) ventilation, particularly early nCPAP (14,22). This could be due to longer periods of mechanical ventilation, less stable oxygen delivery, increased stress, and increased inflammation among other factors. For example, there was a greater degree of fluctuation in FiO2 in both LV-PPV and HFOV animals compared with early (but not delayed) nCPAP (22). The density of astrocytes in the cerebral hemispheres of animals ventilated with HFOV and LV-PPV was 40% higher than with nCPAP (22), suggesting that there might be a greater level of underlying neuronal stress. In the cerebellum, both LV-PPV and HFOV ventilation seemed to have an adverse affect on granule cell neurogenesis not observed with either early or delayed nCPAP (14). We suggest that this might be a consequence of the sensitivity of cell division to fluctuations in oxygen and/or inflammation.
In conclusion, early sustained HFOV compared with LV-PPV did not increase the risk of brain injury and/or altered brain development in prematurely delivered baboons. Both ventilatory regimens were associated with an overall decrease in the brain growth; there was a tendency for HFOV to minimize the adverse effects. In all prematurely delivered animals, subtle neuropathologies were observed in the forebrain and cerebellum. Such adverse effects on brain morphology could contribute to neurodevelopmental delay or sensory and motor impairments in postnatal life.
Abbreviations
- dg:
-
days of gestation
- EGL:
-
external granule layer
- FiO2:
-
fraction of inspired oxygen
- H&E:
-
hematoxylin and eosin
- GFAP:
-
glial fibrillary acidic protein
- HFOV:
-
high-frequency oscillatory ventilation
- Iba1:
-
ionized calcium-binding adapter molecule 1
- IGL:
-
inner granule layer
- IR:
-
immunoreactivity
- LV-PPV:
-
low tidal volume positive pressure ventilation
- MBP:
-
myelin basic protein
- ML:
-
molecular layer
- SFI:
-
surface folding index
- WM:
-
white matter
References
Bodeau-Livinec F, Marlow N, Ancel PY, Kurinczuk JJ, Costeloe K, Kaminski M 2008 Impact of intensive care practices on short-term and long-term outcomes for extremely preterm infants: comparison between the British Isles and France. Pediatrics 122: e1014–e1021
Marlow N, Wolke D, Bracewell MA, Samara M 2005 Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med 352: 9–19
Yoder BA, Siler-Khodr T, Winter VT, Coalson JJ 2000 High-frequency oscillatory ventilation: effects on lung function, mechanics, and airway cytokines in the immature baboon model for neonatal chronic lung disease. Am J Respir Crit Care Med 162: 1867–1876
McCulloch PR, Forkert PG, Froese AB 1988 Lung volume maintenance prevents lung injury during high frequency oscillatory ventilation in surfactant-deficient rabbits. Am Rev Respir Dis 137: 1185–1192
Thome UH, Carlo WA, Pohlandt F 2005 Ventilation strategies and outcome in randomised trials of high frequency ventilation. Arch Dis Child Fetal Neonatal Ed 90: F466–F473
Henderson-Smart DJ, Cools F, Bhuta T, Offringa M 2007 Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database Syst Rev CD000104
Moriette G, Paris-Llado J, Walti H, Escande B, Magny JF, Cambonie G, Thiriez G, Cantagrel S, Lacaze-Masmonteil T, Storme L, Blanc T, Liet JM, Andre C, Salanave B, Breart G 2001 Prospective randomized multicenter comparison of high-frequency oscillatory ventilation and conventional ventilation in preterm infants of less than 30 weeks with respiratory distress syndrome. Pediatrics 107: 363–372
1989 High-frequency oscillatory ventilation compared with conventional mechanical ventilation in the treatment of respiratory failure in preterm infants. The HIFI Study Group. N Engl J Med 320: 88–93
Johnson AH, Peacock JL, Greenough A, Marlow N, Limb ES, Marston L, Calvert SA 2002 High-frequency oscillatory ventilation for the prevention of chronic lung disease of prematurity. N Engl J Med 347: 633–642
Marlow N, Greenough A, Peacock JL, Marston L, Limb ES, Johnson AH, Calvert SA 2006 Randomised trial of high frequency oscillatory ventilation or conventional ventilation in babies of gestational age 28 weeks or less: respiratory and neurological outcomes at 2 years. Arch Dis Child Fetal Neonatal Ed 91: F320–F326
Truffert P, Paris-Llado J, Escande B, Magny JF, Cambonie G, Saliba E, Thiriez G, Zupan-Simunek V, Blanc T, Roze JC, Breart G, Moriette G 2007 Neuromotor outcome at 2 years of very preterm infants who were treated with high-frequency oscillatory ventilation or conventional ventilation for neonatal respiratory distress syndrome. Pediatrics 119: e860–e865
Dieni S, Inder T, Yoder B, Briscoe T, Camm E, Egan G, Denton D, Rees S 2004 The pattern of cerebral injury in a primate model of preterm birth and neonatal intensive care. J Neuropathol Exp Neurol 63: 1297–1309
Coalson JJ, Winter VT, Siler-Khodr T, Yoder BA 1999 Neonatal chronic lung disease in extremely immature baboons. Am J Respir Crit Care Med 160: 1333–1346
Rees SM, Loeliger MM, Munro KM, Shields A, Dalitz PA, Dieni S, Thomson MA, Coalson J, Inder T 2009 Cerebellar development in a baboon model of preterm delivery: impact of specific ventilatory regimes. J Neuropathol Exp Neurol 68: 605–615
Rees SM, Camm EJ, Loeliger M, Cain S, Dieni S, McCurnin D, Shaul PW, Yoder B, McLean C, Inder TE 2007 Inhaled nitric oxide: effects on cerebral growth and injury in a baboon model of premature delivery. Pediatr Res 61: 552–558
Van Essen DC 1997 A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 385: 313–318
Gaglioti P, Danelon D, Bontempo S, Mombro M, Cardaropoli S, Todros T 2005 Fetal cerebral ventriculomegaly: outcome in 176 cases. Ultrasound Obstet Gynecol 25: 372–377
Ganat Y, Soni S, Chacon M, Schwartz ML, Vaccarino FM 2002 Chronic hypoxia up-regulates fibroblast growth factor ligands in the perinatal brain and induces fibroblast growth factor-responsive radial glial cells in the sub-ependymal zone. Neuroscience 112: 977–991
Benveniste EN 1997 Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J Mol Med 75: 165–173
John GR, Lee SC, Song X, Rivieccio M, Brosnan CF 2005 IL-1-regulated responses in astrocytes: relevance to injury and recovery. Glia 49: 161–176
Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ 2003 Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr 143: 171–179
Loeliger M, Inder T, Cain S, Ramesh RC, Camm E, Thomson MA, Coalson J, Rees SM 2006 Cerebral outcomes in a preterm baboon model of early versus delayed nasal continuous positive airway pressure. Pediatrics 118: 1640–1653
Lordkipanidze T, Dunaevsky A 2005 Purkinje cell dendrites grow in alignment with Bergmann glia. Glia 51: 229–234
Hayes TL, Lewis DA 1993 Hemispheric differences in layer III pyramidal neurons of the anterior language area. Arch Neurol 50: 501–505
Limperopoulos C, Soul JS, Gauvreau K, Huppi PS, Warfield SK, Bassan H, Robertson RL, Volpe JJ, du Plessis AJ 2005 Late gestation cerebellar growth is rapid and impeded by premature birth. Pediatrics 115: 688–695
Acknowledgements
We thank Dr Jaqueline Coalson, Ms Vicki Winter, and staff at the Bronchopulmonary Dysplasia Resource Centre, San Antonio, TX, for provision of baboon tissue and Ms Kathryn Munro for histologic assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by NIH grants R01 HL074942, HL52636 (in part), and HL52646 (in part).Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.pedresearch.org).
Rights and permissions
About this article
Cite this article
Loeliger, M., Inder, T., Shields, A. et al. High-Frequency Oscillatory Ventilation Is Not Associated With Increased Risk of Neuropathology Compared With Positive Pressure Ventilation: A Preterm Primate Model. Pediatr Res 66, 545–550 (2009). https://doi.org/10.1203/PDR.0b013e3181bb0cc1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e3181bb0cc1
This article is cited by
-
Hippocampal epigenetic and insulin-like growth factor alterations in noninvasive versus invasive mechanical ventilation in preterm lambs
Pediatric Research (2021)
-
Weak functional connectivity in the human fetal brain prior to preterm birth
Scientific Reports (2017)
-
Mitochondrial dysfunction in alveolar and white matter developmental failure in premature infants
Pediatric Research (2017)
-
MR imaging correlates of white-matter pathology in a preterm baboon model
Pediatric Research (2012)
-
The Instrumented Fetal Sheep as a Model of Cerebral White Matter Injury in the Premature Infant
Neurotherapeutics (2012)