Abstract
We investigated return of spontaneous circulation and of cerebral oxygenation after asphyxia-induced cardiac arrest, using ventilation with air, throughout, or with 100% oxygen for a shorter or longer period. Arterial pressure, heart rate, regional cerebral oxygen saturation (CrSO2), and brain tissue oxygen tension (PbtO2) were measured in 1-d-old piglets that were hypoventilated with air and left in apnea until cardiac arrest. They were randomly assigned to be resuscitated with air (n = 13), or with oxygen for 3 (n = 12) or 30 min (n = 13) and then with air. Nine, 10, and 10 animals, respectively, needed closed chest cardiac massage. One, none, and one, respectively, died. Median (quartile range) times from start of ventilation until heart rate reached 150 bpm were 67 (60–76), 88 (76–126), and 68 (56–81) s. They were not significantly different, nor were the arterial pressure responses, times until CrSO2 reached 30%, or times until PbtO2 had increased by 0.1 kPa from its nadir. Peak PbtO2 values during resuscitation were 4.2 (3.3–5.4), 12 (6.4–15), and 25 (15–36) kPa. Thus, pure oxygen did not accelerate the recovery of circulation or of cerebral oxygenation, while even a brief exposure caused cerebral hyperoxia.
Similar content being viewed by others
Main
The optimal fraction of inspired O2 (FiO2) for resuscitation of asphyxiated neonates is still not settled, although clinical and experimental evidences indicate that 21% is superior to 100% (1–4). In most of these studies, the time of exposure to pure oxygen exceeded 5 min, and it is unknown whether a very brief exposure to high FiO2 at the start of resuscitation might improve myocardial oxygenation and hasten the return of adequate heart function without causing an organ damage.
In three studies of experimental asphyxia (5–7), arterial pressure was restored as quickly by air ventilation as with 100% oxygen. However, few of the animals in these studies needed closed chest cardiac massage (CCCM), and the possibility remains that subjects with asphyxia, so severe that CCCM is needed, might be easier to resuscitate with a high FiO2. We tested this in 1-d-old piglets in cardiac arrest caused by severe asphyxia and hypothesized that ventilation with 100% oxygen would restore heart rate (HR) and arterial pressure faster than would ventilation with air. In addition, return of cerebral oxygenation was analyzed, by measuring regional oxygen saturation (CrSO2) and brain tissue oxygen tension (PbtO2). The asphyxia was induced by hypoventilation followed by apnea to achieve the high blood Pco2 levels that are characteristic of severe clinical perinatal asphyxia (8).
Because the PbtO2 is relevant to the question whether a high FiO2 after asphyxia might cause brain damage, we extended the recording of brain oxygenation beyond the immediate resuscitation phase. Ventilation with 100% oxygen was limited to 3 min in some animals to see whether this could avoid cerebral hyperoxia.
METHODS
This study was approved by the Animal Ethics Research Committee of Lund University. The animals were cared for and handled in accordance with European Guidelines for Use of Experimental Animals.
Animal preparation.
Thirty-eight domestic piglets (12- to 36-h old) were premedicated with intramuscular ketamine (3 mg) and midazolam (0.4 mg). They were weighed and placed in an open heated incubator (Dräger, Lubeck, Germany) where they were kept at normal temperature (38.5–40.0°C), as assessed with a rectal probe. Pulse oximetric saturation was monitored with a probe on the foreleg (OxiMax N-600. Nellcor, Boulder, CO).
An ear vein was cannulated and anesthesia was induced with i.v. propofol (4 mg/kg) and remifentanil (1 μg/kg). After topical laryngeal anesthesia with lidocaine, 10–20 mg, the trachea was intubated with a cuffed tube, and the lungs were mechanically ventilated. The ventilator (Servo 300, Siemens, Sweden) was set in the pressure-regulated volume-controlled mode with the following settings: I/E ratio: 1, FiO2: 0.21, tidal volume: 10 mL/kg, end expiratory pressure: +5 cm H2O, and, initially, ventilator rate: 40/min. Ventilator rate was subsequently adjusted between 30 and 50/min aiming at a Paco2 of 5–6.5 kPa.
Anesthesia was maintained by a continuous infusion of fentanyl, 30 μg · kg−1 · h−1, and midazolam, 0.3 mg · kg−1 · h−1. A solution with 25 g glucose, 70 mmol sodium, 45 mmol chloride, and 25 mmol acetate dissolved per liter was infused at 10 mL · kg−1 · h−1. During the preparation and stabilization phases, 2 mg/kg of vecuronium, a muscular relaxant without anticholinergic effect, was injected i.v. in case of shivering. The right external jugular vein was cannulated and used for drug infusions and fluid replacement. The right and left femoral arteries were cannulated for blood sampling and continuous pressure monitoring (Powerlab monitor, ADInstruments, Hastings, East Sussex, United Kingdom). Three needle electrodes were sutured s.c. to the chest for ECG. The scalp was shaved, and a disposable self-adherent near infrared spectroscopy pediatric probe (Pediatric SomaSensor probe <40 kg, model SPFB-USA, Somanetics Corporation, Troy, MI) was placed over the left scalp and secured with a fabric net. The probe was connected to a cerebral oximeter (INVOS 5100C Cerebral/Somatic Oximeter, Somanetics Corporation) for measurement of CrSO2 by dual wavelength near infrared spectroscopy. The CrSO2 was updated every 6 s and stored in the INVOS Oximeter.
A scalp incision was made to expose the right parietal bone. After drilling a 3-mm diameter hole down to the dura mater, a hollow bolt was screwed into place. The dura mater was perforated using a bevel, a custom made introducer was placed through the bolt, and a flexible microcatheter probe and a thermocouple probe were advanced ∼1 cm into the brain. The microcatheter probe (LICOX; GMS, Mielkendorf, Germany) contained a polarographic oxygen cell. Both probes were connected to a LICOX CMP monitor to obtain temperature-corrected brain tissue Po2 (PbtO2). Measurements were updated every 20 s, and the recordings were stored in a personal computer using the LICOX for PC Software.
Experimental protocol.
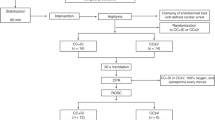
After the preparation, the piglets were allowed to stabilize for at least 30 min, while we ensured that the Paco2 reached target and remained stable for 10 min with fixed ventilator settings. Baseline measurements were then made. Thereafter, vecuronium (2 mg) i.v. was given and asphyxia induced as follows. First, the piglets were hypoventilated with air for 20 min with respiratory rate set to 5/min. Then, ventilation of the lungs was stopped by disconnecting the ventilator. A 60-mL dead-space (Humid-Vent Light, Mediplast AB, Sweden) was connected to the endotracheal tube to prevent diffusion of room air into the lungs. Apnea was maintained until cardiac arrest, which was defined as a HR <50 beats per minute (bpm) with mean arterial pressure <25 mm Hg. Ventilation was resumed one minute after cardiac arrest, and the piglet was randomly assigned to one of three groups: resuscitation with air (n = 13), with 100% oxygen for 3 min and then with air (n = 12), or with 100% oxygen for 30 min and then with air (n = 13). The ventilator settings were the same as at baseline except, possibly, the FiO2. In some animals, cardiac arrest had not developed after 12 min of apnea, in which case apnea was discontinued and resuscitation was carried out as in the other animals, after random assignment to one of the groups.
The arterial pressure and ECG were assessed by an observer, unaware of the group assignment. If no adequate circulation was present after 30 s of ventilation, i.v. epinephrine (10 μg/kg) followed by 30 s of CCCM was given. The arterial pressure was evaluated during the subsequent 30 s, and if circulation had not recovered, a second attempt with epinephrine and 30 s of CCCM was done. If this, too, was unsuccessful, resuscitation ceased. To prevent atelectasis from anesthesia and muscle paralysis, a lung recruitment maneuver was performed at 3, 30, 45, 105, 165, and 225 min after resumption of ventilation, using pressure-controlled ventilation for 15 s with an inspiratory pressure of 30 cm H2O and an end expiratory pressure PEEP of 20 cm H2O.
Arterial samples were taken at baseline, at the end of asphyxia, i.e., just before resumption of ventilation, and after 2.5, 10, 29.5, 60, 120, and 240 min of ventilation, respectively. They were analyzed for Po2, Pco2, pH, and base excess (BE) on an ABL 700 blood-gas analyzer (Radiometer, Copenhagen, Denmark) with settings adjusted to porcine blood according to a factory-installed algorithm.
The piglets were cared for 240 min after resumption of ventilation.
Assessment of immediate circulatory recovery and recovery of cerebral oxygenation.
The speed of circulatory recovery was assessed by measuring the time from start of ventilation until spontaneous HR had increased to 150 bpm. In addition, median curves depicting the early HR and MAP responses were obtained by aligning the individual curves so that the exact times when HR reached 150 bpm coincided. This way, the median curves reflected the typical abruptness with which HR and MAP increased once resuscitation was successful. At any point of time on the curves, data from subjects with ongoing CCCM were excluded from the analysis.
Speed of recovery of cerebral oxygenation was evaluated as the time when CrSO2 reached 30% and time when PbtO2 had increased 0.1 kPa from its nadir. They were obtained to the nearest second, through interpolation.
Analysis of cerebral oxygenation during the first 60 min after resumption of ventilation.
Curves depicting group medians of CrSO2 and PbtO2 at any given time after resumption of ventilation were drawn. In addition, we assessed in each pig maximal (peak) CrSO2 and PbtO2 and the times when these occurred.
Statistics.
Between-group differences were assessed by one-way ANOVA on ranks using Sigmastat version 3.1 (Systat Software GmbH, Ekrath, Germany). In case of significance, Dunn's test was used to assess pairwise differences between groups. p values <0.05 were considered significant. Values in the text and tables are given as median (quartile range) and were calculated using Microsoft Office Excel 2003 (Microsoft, Redmond, WA).
RESULTS
Demographics are shown in Table 1 and brain oxygenation, MAP, HR, and blood gases at baseline in Tables 1 and 2.
The concluding apneic phase of the asphyxia lasted 6 min (5–10 min) [median (quartile range) for all animals] and in the last 30 s of apnea, MAP was 14 mm Hg (11–19 mm Hg) and HR 12 bpm (2–36 bpm). CrSO2 was 15%, the minimum values displayed by the equipment, and PbtO2 was 0.0 kPa (0.0–0.1 kPa). None of these measures differed between groups.
In two animals, subsequently resuscitated with air, and in one, subsequently given pure oxygen for 30 min, apnea was discontinued after 12 min without our criteria for cardiac arrest being met. HR in these was 90, 60, and 70 bpm and MAP 47, 19, and 17 mm Hg.
Immediate outcome of resuscitation.
The number of pigs randomized to resuscitation with air, 3 min of oxygen, and 30 min of oxygen, were 13, 12, and 13, respectively. Those pigs requiring CCCM after the initial 30 s of resumed ventilation counted 9, 10, and 10, respectively. Of these, two, two, and one pigs required a second and final attempt with CCCM, which failed in one, zero, and zero pigs. Once effective heart action was being reestablished, HR increased very quickly in all three groups: from below 100 bpm to over 200 bpm within 15 s. The pattern was similar for MAP (Fig. 1). One piglet allotted to ventilation with 30 min of oxygen was initially successfully resuscitated, but died at 10 min after resumption of ventilation. There were no significant between-group differences in respect of time to reach a HR of 150 bpm, time until CrSO2 reached 30%, or time until PbtO2 had increased by 0.1 kPa from its nadir (Table 3).
Speed of return of spontaneous circulation among survivors. The curves show group medians of heart rate and arterial pressure at any given time before or after the point when heart rate exceeded 150 bpm. The right part of the diagram details the first 3 min. Groups as follows: air (n = 12, solid thick line), 3 min of oxygen (n = 12, dotted line), and 30 min of oxygen (n = 12, solid thin line).
Blood gases and cerebral oxygenation after resumption of ventilation.
In the groups initially ventilated with 100% oxygen after asphyxia, marked arterial hyperoxemia 34 kPa (30–41 kPa) was seen already by 2.5 min (Table 2), and the median Pao2 remained at this or a higher level until switching to air. PbtO2 peaked at 25 kPa (15–36 kPa), and CrSO2 at 92% (86–94%) in piglets ventilated with 100% oxygen for 30 min. The corresponding figures among those ventilated with air only were 4.2 kPa (3.3–5.4 kPa), and 68% (61–72%), that is ∼2.2 kPa and 23% more, respectively, than at baseline (Table 1). The group ventilated with 100% oxygen for 3 min was intermediate in respect of peak PbtO2 and peak CrSO2: 12 kPa (6.4–15 kPa) and 80% (73–87%). The time to peak PbtO2 was 12 min (9–14 min) in the air-resuscitated group and 11 min (10–12 min) in the 30 min of oxygen group. In the group ventilated with 100% oxygen for 3 min, PbtO2 reached its peak at 7 min (7–11 min) and then rapidly decreased to the same levels as in the air ventilated group (Fig. 2). CrSO2 in the same group peaked already at 202 s (180–204 s) (Fig. 2). Paco2 decreased on resumed ventilation but was over 8 kPa in most pigs at 10 min.
Brain tissue oxygen tension among survivors (A). Curves depict group medians in piglets resuscitated with air (n = 12, solid thick line), or with 3 or 30 min of 100% oxygen followed by air (n = 12, dotted line and n = 12 solid thin line, respectively). On the right, findings during the first 3 min of resumed ventilation are shown at increased resolution. Cerebral regional oxygen saturation (B). The minimum value displayed by the measurement equipment was 15% (see text for other explanations).
At 60 min, group medians for PbtO2 and CrSO2 (Fig. 2) were similar to baseline (Table 1), as were Paco2 and Pao2 (Table 2), which remained unchanged throughout the rest of the study. In contrast, arterial BE and pH at 60 min were still below baseline (Table 2). At 240 min, BE of all animals was +5 mmol/L (1–6 mmol/L), pH 7.45 (7.39–7.47), HR 148 bpm (127–179 bpm), and MAP 54 mm Hg (46–60 mm Hg), There was, thus, a slight over-shoot in BE, while pH, HR, and MAP were at the same levels as at baseline (Tables 1 and 2). There was no significant difference between groups at 60 or 240 min, in respect of any of these measures.
DISCUSSION
The main finding of this study is that ventilation with 100% oxygen, instead of air, did not accelerate the return of adequate spontaneous circulation in newborn piglets requiring CCCM for resuscitation from severe asphyxia. Moreover, oxygen ventilation gave very high levels of PbtO2, otherwise seen only under exposure to hyperbaric oxygen (9) and in our previous study on lambs (6).
The equally fast circulatory recovery with air as with oxygen is in line with results of Fugelseth et al. (10) showing that 100% oxygen did not decrease myocardial injury as measured by the release of cardiac troponin I (cTnI) in hypoxic piglets. This study differs from theirs, however, in that we studied recovery from cardiac arrest, whereas their animals maintained both HR and cardiac output during the hypoxic event. Another difference is that their animals remained normocarbic, while ours were markedly hypercarbic, a characteristic of severe clinical asphyxia (8).
In this study, PbtO2 peaked at 12 kPa (6.4–15 kPa) and 25 kPa (15–36 kPa) in piglets resuscitated with oxygen for 3 and 30 min, respectively, whereas in the air ventilated group, the corresponding value was only 4.2 kPa (3.3–5.4 kPa). In contrast, Lyng et al. (11) reported a peak PbtO2 around only 5 kPa in piglets resuscitated with 100% oxygen for 30 min after a hypoxic injury with normal Paco2. We believe that the discrepancies between the studies may largely be explained by the much higher Paco2 at the end of asphyxia in this study: 21 kPa (19–23 kPa), and in the study of lambs (6). Although peak PbtO2 in the piglets that we ventilated with oxygen for 30 min was remarkably high, it was, nevertheless, only about half that in the lambs, perhaps because Paco2 decreased more slowly in the latter. Other possible explanations for the divergent findings are differences in placement of the PbtO2 electrode, degree of injury, and species.
We conclude that ventilation with air restored the circulation as fast as did ventilation with oxygen in piglets resuscitated after asphyxia-induced cardiac arrest. Thus, resuscitation with an FiO2 of 0.21 should be adequate even in very severe asphyxia, provided the lungs are normal and easy to ventilate. Furthermore, prolonged oxygen ventilation resulted in very high brain tissue oxygen tensions, emphasizing the need to limit oxygen exposure in asphyxiated neonates.
Abbreviations
- BE:
-
base excess
- Bpm:
-
beats per minute
- CCCM:
-
closed chest cardiac massage
- CrSO2:
-
regional cerebral oxygen saturation
- FiO2:
-
inspired fraction of oxygen
- HR:
-
heart rate
- MAP:
-
mean arterial pressure
- PbtO2:
-
brain tissue partial pressure of oxygen
References
Saugstad OD, Rootwelt T, Aalen O 1998 Resuscitation of asphyxiated newborn infants with room air or oxygen: an international controlled trial: the Resair 2 study. Pediatrics 102: e1
Vento M, Asensi M, Sastre J, Garcia-Sala F, Pallardo FV, Vina J 2001 Resuscitation with room air instead of 100% oxygen prevents oxidative stress in moderately asphyxiated term neonates. Pediatrics 107: 642–647
Vento M, Asensi M, Sastre J, Lloret A, Garcia-Sala F, Vina J 2003 Oxidative stress in asphyxiated term infants resuscitated with 100% oxygen. J Pediatr 142: 240–246
Saugstad OD, Ramji S, Vento M 2005 Resuscitation of depressed newborn infants with ambient air or pure oxygen: a meta-analysis. Biol Neonate 87: 27–34
Markus T, Hansson S, Amer-Wahlin I, Hellstrom-Westas L, Saugstad OD, Ley D 2007 Cerebral inflammatory response after fetal asphyxia and hyperoxic resuscitation in newborn sheep. Pediatr Res 62: 71–77
Perez-de-Sa V, Cunha-Goncalves D, Nordh A, Hansson S, Larsson A, Ley D, Fellman V, Werner O 2009 High brain tissue oxygen tension during ventilation with 100% oxygen after fetal asphyxia in newborn sheep. Pediatr Res 65: 57–61
Borke WB, Munkeby BH, Morkrid L, Thaulow E, Saugstad OD 2004 Resuscitation with 100% O(2) does not protect the myocardium in hypoxic newborn piglets. Arch Dis Child Fetal Neonatal Ed 89: F156–F160
Engle WD, Laptook AR, Perlman JM 1999 Acute changes in arterial carbon dioxide tension and acid-base status and early neurologic characteristics in term infants following perinatal asphyxia. Resuscitation 42: 11–17
van Hulst RA, Haitsma JJ, Klein J, Lachmann B 2003 Oxygen tension under hyperbaric conditions in healthy pig brain. Clin Physiol Funct Imaging 23: 143–148
Fugelseth D, Borke WB, Lenes K, Matthews I, Saugstad OD, Thaulow E 2005 Restoration of cardiopulmonary function with 21% versus 100% oxygen after hypoxaemia in newborn pigs. Arch Dis Child Fetal Neonatal Ed 90: F229–F234
Lyng K, Braakhuis M, Froen JF, Stray-Pedersen B, Saugstad OD 2005 Inflammation increases vulnerability to hypoxia in newborn piglets: effect of reoxygenation with 21% and 100% O2 . Am J Obstet Gynecol 192: 1172–1178
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by The Laerdal Foundation, Lund University Hospital, and Lund University Medical Faculty grants.
Rights and permissions
About this article
Cite this article
Linner, R., Werner, O., Perez-de-Sa, V. et al. Circulatory Recovery Is as Fast With Air Ventilation as With 100% Oxygen After Asphyxia-Induced Cardiac Arrest in Piglets. Pediatr Res 66, 391–394 (2009). https://doi.org/10.1203/PDR.0b013e3181b3b110
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e3181b3b110
This article is cited by
-
Monitoring of Brain Tissue Oxygen Tension in Cardiac Arrest: a Translational Systematic Review from Experimental to Clinical Evidence
Neurocritical Care (2024)
-
Kindliche Notfälle im Kreißsaal – Teil 1
Die Gynäkologie (2023)
-
Randomized trial of oxygen weaning strategies following chest compressions during neonatal resuscitation
Pediatric Research (2021)
-
Dynamic changes in arterial blood gas during cardiopulmonary resuscitation in out-of-hospital cardiac arrest
Scientific Reports (2021)
-
Versorgung und Reanimation des Neugeborenen nach der Geburt
Notfall + Rettungsmedizin (2021)