Abstract
A secondary analysis of a randomized study was performed to study the relationship between volumetric capnography (VCAP) and arterial CO2 partial pressure (PCO2) during cardiopulmonary resuscitation (CPR) and to analyze the ability of these parameters to predict the return of spontaneous circulation (ROSC) in a pediatric animal model of asphyxial cardiac arrest (CA). Asphyxial CA was induced by sedation, muscle relaxation and extubation. CPR was started 2 min after CA occurred. Airway management was performed with early endotracheal intubation or bag-mask ventilation, according to randomization group. CPR was continued until ROSC or 24 min of resuscitation. End-tidal carbon dioxide (EtCO2), CO2 production (VCO2), and EtCO2/VCO2/kg ratio were continuously recorded. Seventy-nine piglets were included, 26 (32.9%) of whom achieved ROSC. EtCO2 was the best predictor of ROSC (AUC 0.72, p < 0.01 and optimal cutoff point of 21.6 mmHg). No statistical differences were obtained regarding VCO2, VCO2/kg and EtCO2/VCO2/kg ratios. VCO2 and VCO2/kg showed an inverse correlation with PCO2, with a higher correlation coefficient as resuscitation progressed. EtCO2 also had an inverse correlation with PCO2 from minute 18 to 24 of resuscitation. Our findings suggest that EtCO2 is the best VCAP-derived parameter for predicting ROSC. EtCO2 and VCO2 showed an inverse correlation with PCO2. Therefore, these parameters are not adequate to measure ventilation during CPR.
Similar content being viewed by others
Introduction
Capnography is a useful monitoring technique in cardiopulmonary resuscitation (CPR)1. It has multiple applications that include verifying adequate endotracheal tube position and assessing quality of chest compressions in adult CPR2,3. Moreover, a retrospective study that analyses 426 out-of-hospital cardiac arrest cases suggest an association between capnographic values and return of spontaneous circulation (ROSC)4.
Regarding pediatric CPR, there is insufficient evidence supporting the use of end-tidal carbon dioxide (EtCO2) as a as a marker of quality or as a prognostic marker during CPR. Therefore, the European Resuscitation Council guidelines5 advocate its use to monitor ETT position and state that it can help to rapidly detect ROSC, but do not recommend it as a marker of CPR quality, while the American Heart Association Guidelines6 state that EtCO2 monitoring may be considered to assess the quality of chest compressions7.
Time-based capnography, widely accessible, provides valuable information such as end-tidal carbon dioxide (EtCO2) values and capnography wave analysis. Other techniques as volumetric capnography (VCAP) offer a representation of CO2 production, transport and elimination, that could be useful during CPR, although they might not be always available in a CPR setting8. However, there are no published studies regarding the relationship between VCAP-derived parameters and real ventilation status (based on PCO2) in pediatric animal models of cardiac arrest (CA).
We conducted a secondary analysis of a previously published experimental study using a pediatric animal model of asphyxial cardiac arrest9, to analyze the relationship between VCAP-derived parameters and arterial blood CO2 partial pressure (PCO2) and their ability to predict ROSC.
Methods
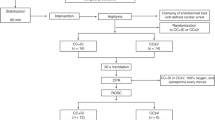
This study is a secondary analysis of the data obtained in a randomized prospective experimental study, performed in the Experimental Medicine and Surgery Department of a third level hospital in Madrid, Spain. The experimental protocol was approved by the Local Ethics Committee in Animal Research and authorized by the Autonomous Community of Madrid (reference number PROEX 096/19). The study was developed in compliance with the ARRIVE guidelines, and all methods were carried out in accordance with guidelines and regulations. The study protocol has already been thoroughly described in a previously published article9.
Animal preparation and monitoring
Seventy-nine 3-month-old miniature pigs, weighing 9–12 kg, were included.
Arterial, peripheral and central venous lines were inserted and connected to a PiCCO system for hemodynamic monitoring (heart rate, arterial blood pressure cardiac index and temperature). ECG and pulse oximetry were monitored continuously. Cerebral (ScO2) and splanchnic (SsO2) oxygen saturations were monitored by near-infrared spectroscopy (NIRS) (INVOS Cerebral Oximeter monitor, Somanetics, Troy, Michigan, USA). Respiratory parameters were continuously monitored using a sensor placed at the Y piece and connected to a Respironics NM3 monitor (Philips Healthcare, Markham, ON, Canada). Maintenance fluids containing glucose and saline were infused. Animal temperature was kept between 37 and 39 °C. Chest compressions were performed guiding depth and rate with a defibrillator monitor (Zoll Z series).
Experimental protocol
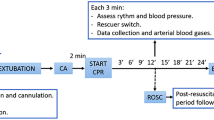
After instrumentation, sedation and an initial stabilization period, an asphyxial CA was induced by extubating animals after administering an intravenous dose of atracurium. CA was defined as a mean arterial pressure under 25 mmHg. Advanced CPR was started 2 min after diagnosing CA.
Animals were randomized in five different groups according to airway management (early intubation-ETI or bag-mask ventilation-BMV) and delivery of ventilation during CPR: with real-time tidal volume feedback (TVF) of 7 or 10 ml/kg, or without feedback, depending on chest expansion (standard ventilation—SV). Resuscitation groups are shown in Table 1. Resuscitation was continued until ROSC or up to a maximum of 24 min.
Study variables
Clinical and monitoring parameters were collected at baseline, 5 min after extubation, before the start of CPR and every 3 min during resuscitation. Arterial blood gases were withdrawn at baseline and after every 3 min of CPR. Each ventilation was recorded, and respiratory parameters were registered at baseline and every 3 min during CPR.
Statistical analysis
The SPSS statistical package, version 25.0 (SPSS Inc, Chicago, USA) was used for statistical analysis. Continuous variables are expressed as means with standard deviation and categorical variables as percentages. The correlation between continuous variables was calculated with Pearson's correlation coefficient. To assess the sensitivity and specificity of the different variables to detect the return of spontaneous circulation, a ROC (receiver operating characteristic) curve was performed. The area under the curve and the Youden index were calculated. p values less than 0.05 were considered significant.
A sample size calculation was performed accepting an alpha risk of 0.05 and a beta risk of 0.2. ROSC was considered the main effect, with an estimated incidence of 30% based on previous experiences10,11,12 with the same animal model. An effect size of 25% on the usual incidence of ROSC was considered significant. A drop-out rate of 10% was estimated. Therefore, 15 animals per group were required, with a total number of 75 animals.
Results
Seventy-nine piglets weighting 11.3 ± 1.2 kg were included in the study, with a ROSC rate of 32.9% (26 animals). ROSC rate according to randomization group is shown in Table 1.
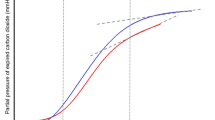
A receiver operator characteristic (ROC) curve that included EtCO2, VCO2, VCO2/kg and ratio EtCO2/VCO2/kg was performed, showing that EtCO2 at 3 min of resuscitation was the only predictor of ROSC, with an AUC of 0.71 (Table 2, Fig. 1). The optimal cut-off point for predicting ROSC was an EtCO2 value of 21.58 mmHg, with a sensitivity of 56% and a specificity of 89%. Sensitivity, specificity and Youden’s index for predicting ROSC of EtCO2 values of 10, 15, 20 and 25 mmHg are shown in Table 3.
Animals that achieved ROSC had higher median EtCO2 levels (30.1 vs 11.9 mmHg) than those who did not, regardless of airway management (intubation or bag-mask ventilation) after applying a linear multiple regression model (p < 0.01).
Linear correlation was performed to study the relationship between PCO2 and EtCO2, VCO2, VCO2/kg and ratio EtCO2/VCO2/kg (Table 4). A negative correlation was found between EtCO2 and PCO2 from resuscitation minutes 18 to 24. VCO2 and VCO2/kg had a statistically significant negative correlation with PCO2 during resuscitation, with more negative correlation as resuscitation time increased. Regarding EtCO2/VCO2/kg ratio, it was only significantly correlated with PCO2 at resuscitation minute 3.
Discussion
This study is, as far as we know, the first experimental animal study that analyses the relationship between VCAP-derived parameters, survival, and ventilation status during pediatric cardiopulmonary resuscitation.
Our results show that EtCO2 is the best capnometric parameter to predict ROSC, which has already been demonstrated in previous publications4,13,14,15,16,17,18. We found that animals that achieved ROSC had higher median EtCO2 levels than non-survivors and we obtained an optimal EtCO2 cutoff point of 21.58 mmHg to predict ROSC.
A large cohort study that analyzed data of 143 pediatric cardiac arrest events18 reported a significant difference in median EtCO2 between events that achieved ROSC and those that did not. However, when data were stratified based on patient age, this relationship was only significant in adolescents, without differences in EtCO2 levels in children and infants that achieved ROSC and those who did not survive.
Several authors have analyzed EtCO2 cutoff points for predicting ROSC: Sorcher et al.18 used a cutoff point of 20 mmHg. Chalak et al.14 established an EtCO2 cutoff point of 14 mmHg in an experimental model of neonatal asphyxial CA, while Stine et al.15 reviewed CA episodes in patients less than 6 months hospitalized in an intensive care unit, setting an EtCO2 cutoff point of 17–18 mmHg. Both studies defined ROSC as the achievement of a heart rate higher than 60 beats per minute. Another study of in-hospital CA in adults set an EtCO2 cutoff point of 25.5 mmHg for predicting sustained ROSC16.
Given these differences between studies and the number of influencing factors (hyperventilation, drug administration, etc.)1, it is specially challenging to stablish an absolute EtCO2 cutoff point for predicting ROSC. Therefore, a case–control study of adult out-of-hospital CA suggested that CO2 trends were more useful to predict ROSC, as survivors had more positive trends than non-survivors19.
Some authors suggested that VCO2 and VCO2/kg were also good predictors of ROSC17,20,21, although these parameters did not demonstrate utility for this purpose in our study. Most of these studies induced a ventricular-fibrillation cardiac arrest. Nevertheless, cardiac arrest of sudden cardiac origin (such as ventricular fibrillation) is not associated with high CO2 levels at the beginning of CPR, as in CA of respiratory origin22,23,24. This fact could explain the different performance of VCO2 and VCO2/kg in our study. However, another experimental study of asphyxial cardiac arrest in neonatal piglets found that both EtCO2 and VCO2 during CPR were higher in survivors25.
Regarding the relationship between EtCO2 and PCO2, we found an inverse correlation from minute 18 to minute 24 of CPR. It would be expected that changes in PCO2 would be associated with parallel changes in EtCO2. Nevertheless, EtCO2 is greatly affected by pulmonary blood flow (as with decreased cardiac output), which can explain the negative correlation.
These findings were consistent with a previous study showing a progressive increase of PCO2 and decrease of EtCO2 throughout CPR14. Another study in out of hospital CA in adult patients, however, showed moderate correlation between EtCO2 and PCO2 during reanimation. Nevertheless, blood samples in that study were obtained at any point of CPR when an arterial line was obtained, regardless of the duration of CA or resuscitation26.
Zhang et al. found that EtCO2 was a useful parameter to predict ROSC, and that VCO2/kg ratio had similar capacity to predict ROSC in an experimental porcine cardiac arrest model20. This group also analyzed the relationship between EtCO2/VCO2/kg and ventilation during CPR in an experimental study with adult pigs, finding that this ratio showed good performance in discriminating hyperventilation from non-hyperventilation13.
Regarding VCO2 and VCO2/kg, our results show an inverse correlation of both parameters with PCO2, which became more significant as resuscitation progressed in time. VCO2 values are determined by tidal volume and EtCO2. Therefore, if tidal volume remains stable, VCO2 will be affected by the same factors that affect EtCO2: the decrease of cardiac output and pulmonary perfusion during CPR modifies ventilation/perfusion ratio, reducing expired CO2 fraction, and consequently, VCO2.
As for EtCO2/VCO2/kg ratio, we only found correlation with PCO2 at minute 3 of resuscitation. Zhang et al.20 reported that this parameter was a good predictor of hyperventilation, although PCO2 was not measured during resuscitation.
VCO2 measurement requires the use of a specific volumetric capnograph. Such capnographs are not widely available and, according to our results, this parameter does not accurately reflect ventilation during CPR. In the light of these findings, VCAP parameters do not seem to offer any additional benefits than regular capnography during CPR. However, these findings should be validated with specific pediatric clinical studies.
Our study has several limitations. Although we used a validated pediatric animal model for this purpose, the results from animal experiments cannot be directly extrapolated to children. Besides, the variability of VCAP measurement when using an endotracheal tube or a face mask could interfere with the results.
Conclusions
In an experimental model of pediatric asphyxial cardiac arrest, EtCO2 was the only VCAP-derived parameter for predicting ROSC. VCO2 and VCO2/kg and EtCO2 had an inverse correlation with PCO2 during CPR. Therefore, they are not suitable for assessing PCO2 during resuscitation. These findings highlight the importance of measuring arterial CO2 partial pressure during CPR.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Sandroni, C., De, S. P. & Arrigo, S. D. Capnography during cardiac arrest. Resuscitation 132, 73–77 (2018).
Soar, J. et al. European Resuscitation Council Guidelines 2021: Adult advanced life support. Resuscitation 161, 115–151 (2021).
Panchal, A. R. et al. Part 3: Adult basic and advanced life support: 2020 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 142, 366–468 (2020).
Elola, A. et al. Capnography: A support tool for the detection of return of spontaneous circulation in out-of-hospital cardiac arrest. Resuscitation 142, 153–161 (2019).
Van de Voorde, P. et al. European resuscitation council guidelines 2021: Paediatric life support. Resuscitation 161, 327–387 (2021).
Topjian, A. A. et al. Part 4: Pediatric basic and advanced life support: 2020 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 142, 469–523 (2020).
Lauridsen, K. G., Raymond, T. & Sutton, R. M. Hemodynamic-directed pediatric cardiopulmonary resuscitation using ET-CO2: Are physiologic targets really patient Centric?. Resuscitation 170, 324–326 (2022).
Suarez-Sipmann, F., Bohm, S. H. & Tusman, G. Volumetric capnography: The time has come. Curr. Opin. Crit. Care 20, 333–339 (2014).
Manrique, G. et al. Effects of airway management and tidal volume feedback ventilation during pediatric resuscitation in piglets with asphyxial cardiac arrest. Sci. Rep. 11, 1–10 (2021).
López, J. et al. Different respiratory rates during resuscitation in a pediatric animal model of asphyxial cardiac arrest. PLoS One 11(9), e0162185 (2016).
López-Herce, J. et al. Hemodynamic, respiratory, and perfusion parameters during asphyxia, resuscitation, and post-resuscitation in a pediatric model of cardiac arrest. Intensive Care Med. 37, 147–155 (2011).
Manrique, G. et al. Quality of chest compressions during pediatric resuscitation with 15:2 and 30:2 compressions-to-ventilation ratio in a simulated scenario. Sci. Rep. 10(1), 6828 (2020).
Zhang, L. et al. New volumetric capnography-derived parameter: A potentially valuable tool for detecting hyperventilation during cardiopulmonary resuscitation in a porcine model. J. Thorac. Dis. 13, 3467–3477 (2021).
Chalak, L. F. et al. End-tidal CO2 detection of an audible heart rate during neonatal cardiopulmonary resuscitation after asystole in asphyxiated piglets. Pediatr. Res. 69, 401–405 (2011).
Stine, C. N. et al. Quantitative end-tidal CO2 can predict increase in heart rate during infant cardiopulmonary resuscitation. Heliyon 5, e01871 (2019).
Wang, A. Y. et al. Initial end-tidal CO2 partial pressure predicts outcomes of in-hospital cardiac arrest. Am. J. Emerg. Med. 34, 2367–2371 (2016).
Xu, J. et al. Modified volumetric capnography-derived parameter: A potentially stable indicator in monitoring cardiopulmonary resuscitation efficacy in a porcine model. Resuscitation 150, 94–101 (2020).
Sorcher, J. L. et al. Association of end-tidal carbon dioxide levels during cardiopulmonary resuscitation with survival in a large paediatric cohort. Resuscitation 170, 316–323 (2022).
Brinkrolf, P. et al. Predicting ROSC in out-of-hospital cardiac arrest using expiratory carbon dioxide concentration: Is trend-detection instead of absolute threshold values the key?. Resuscitation 122, 19–24 (2018).
Zhang, L. et al. Assessment of a new volumetric capnography-derived parameter to reflect compression quality and to predict return of spontaneous circulation during cardiopulmonary resuscitation in a porcine model. J. Clin. Monit. Comput. 36, 199–207 (2022).
Al-Subu, A. M., Hacker, T. A., Eickhoff, J. C., Ofori-Amanfo, G. & Eldridge, M. W. Two-site regional oxygen saturation and capnography monitoring during resuscitation after cardiac arrest in a swine pediatric ventricular fibrillatory arrest model. J. Clin. Monit. Comput. 34, 63–70 (2020).
Berg, R. A. et al. Initial end-tidal CO2 is markedly elevated during cardiopulmonay resuscitation after asphyxial cardiac arrest. Pediatr. Emerg. Care 12, 245–248 (1996).
Bhende, M. S., Karasic, D. G. & Karasic, R. B. End-tidal carbon dioxide changes during cardiopulmonary resuscitation after experimental asphyxial cardiac arrest. Am. J. Emerg. Med. 14, 349–350 (1996).
Lah, K., Križmarić, M. & Grmec, Š. The dynamic pattern of end-tidal carbon dioxide during cardiopulmonary resuscitation: Difference between asphyxial cardiac arrest and ventricular fibrillation/pulseless ventricular tachycardia cardiac arrest. Crit. Care 15, 1–8 (2011).
Li, E. et al. Exhaled CO2 parameters as a tool to assess ventilation-perfusion mismatching during neonatal resuscitation in a swine Model of neonatal asphyxia. PLoS One 11, 1–11 (2016).
Spindelboeck, W. et al. Arterial blood gases during and their dynamic changes after cardiopulmonary resuscitation: A prospective clinical study. Resuscitation 106, 24–29 (2016).
Funding
This work was supported by the PI 18/01632 of Carlos III Health Institute, responsible for Spain’s Health Research and Development Strategy, co-financed by the European Union ERDF funds (European Regional Development Fund), RETICS “Maternal and Child Health and Development Network” (SAMID Network), funded by the PN I+D+i 2013–2016 (Spain), ISCIII-Sub-Directorate General for Research Assessment and Promotion and the European Regional Development Fund (ERDF), ref. RD16/0022 and “Primary Care Interventions to Prevent Maternal and Child Chronic Diseases of Perinatal and Developmental Origin Network” (RICORS), RD21/0012/0011, Instituto de Salud Carlos III, Madrid, Spain. Funders did not participate in the study design, dataset collection and analysis and manuscript writing.
Author information
Authors and Affiliations
Contributions
G.M., J.L.H. and J.U. conceived and designed the study and obtained research funding. S.M.N., G.P., L.B.L., M.G., M.S., R.G., L.H., S.M., J.C., M.J.S., M.J.S.L, A.R.M, D.S., J.L., R.C. and S.N.F. developed the experimental procedures and data collection. G.M. and G.P. managed the data. G.M. and R.G. provided statistical advice on study design and analyzed the data. S.M.N., G.M. and S.N.F. drafted the manuscript, and all authors contributed substantially to its revision. S.M.N., G.M. and J.L.H. take responsibility for the paper as a whole.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de la Mata Navazo, S., Manrique, G., Fernández, S.N. et al. Volumetric capnography and return of spontaneous circulation in an experimental model of pediatric asphyxial cardiac arrest. Sci Rep 13, 12247 (2023). https://doi.org/10.1038/s41598-023-37827-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-37827-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.