Abstract
Adults with inactive Crohn disease have been shown to have normal rates of protein turnover when compared with healthy adults. It is not known whether this is true for adolescents with inactive Crohn disease, when rate of protein synthesis must be greater than that of breakdown for normal development. The objective of this study was to determine whether enteral nutrition acutely suppresses proteolysis and increases protein synthesis in adolescents with inactive Crohn disease. Six adolescents (five males/one female; mean age, 15.8 ± 1.9 y; range, 13.2–17.6 y; mean bone age, 14.6 ± 1.8 y; range, 12.5–17 y) participated. Leucine (Leu) and phenylalanine (Phe) kinetics were measured using stable isotopes under fasted and fed conditions during a single study visit. In response to enteral nutrition, the endogenous rates of appearance (Ra) of Leu and Phe (reflecting proteolysis) decreased significantly by 40%. The percentages of splanchnic uptake of Leu and Phe were 35 ± 10% and 13 ± 12%, respectively. Under fed conditions, utilization of Phe for protein synthesis increased significantly. We conclude that in clinically stable adolescents with Crohn disease, enteral nutrition promotes anabolism by suppressing proteolysis and increasing protein synthesis. Rates of suppression of proteolysis were similar to those reported previously in normal children.
Similar content being viewed by others
Main
Previous studies in children with Crohn disease have shown that growth and accretion of lean body mass are promoted by enteral nutrition (1–3). The rate of total body protein accretion for synthesis of lean body mass is determined by the balance between total body protein breakdown (proteolysis) and protein synthesis. Previous studies of healthy adults and prepubertal children have shown that enteral feeding is associated with significant suppression of whole-body proteolysis, with little change in rates of protein synthesis (4–7). Adults with quiescent Crohn disease have been shown to have normal rates of proteolysis, protein synthesis, and splanchnic uptake of glutamine when compared with healthy adults (8). However, it is not known whether this is true for adolescents with inactive Crohn disease, when net accrual of protein is necessary for increasing lean body mass and the rate of protein synthesis must be greater than that of breakdown.
The primary purpose of this study was to test the hypothesis that enteral nutrition would acutely suppress proteolysis and increase protein synthesis in adolescents with inactive Crohn disease, as this would be expected in healthy adolescents. Studies to examine the balance between proteolysis and protein synthesis in response to nutrition in pediatric patients with Crohn disease are scarce (3,9,10), and no pediatric studies have evaluated the effect of feeding on protein turnover while accounting for splanchnic uptake of Leu and Phe. Based on previous studies in adults with inactive Crohn disease, it was hypothesized that adolescents with inactive Crohn disease would be anabolic, decreasing proteolysis and increasing protein synthesis in response to enteral feeding (8). This hypothesis is supported by reports that Crohn disease remission is associated with improvement of growth and of hormonal and metabolic processes promoting accrual of lean body mass (11–13). However, previous studies have shown that in adolescents with inactive Crohn disease energy expenditure is increased as compared with healthy adolescents (14), and deficits in lean body mass persist during remission (15). These studies suggest that some patients may have difficulty achieving a protein anabolic state, even when seemingly well.
For this pilot study, we used stable isotope methodology to measure (1) the endogenous Ra of the essential amino acids Leu and Phe (reflecting proteolysis), (2) the percentage of splanchnic uptake of these amino acids, (3) the rate of Phe hydroxylation (reflecting catabolism of Phe), and (4) the rate of utilization of Phe for protein synthesis during a basal fasted period and in response to acute administration of enteral nutrition.
SUBJECTS AND METHODS
Study subjects.
This pilot study was approved by the Institutional Review Board of Indiana University School of Medicine and was performed at the General Clinical Research Center. Written informed consent was obtained from the parents of all subjects, and assent was obtained from all participants who served as study subjects. Children and young adults aged 6–21 y with an endoscopic and histologic diagnosis of Crohn disease and no other chronic disease were eligible for study. Six adolescents (five males/one female; mean age, 15.8 ± 1.9 y; range, 13.2–17.6 y; mean bone age, 14.6 ± 1.8 y; range, 12.5–17 y) participated. Characteristics of the six study subjects are summarized in Table 1. All participants had normal serum albumin levels (range, 3.8–4.5 g/dL). Medications for the treatment of Crohn disease that study participants were receiving included infliximab (five participants), mesalamine (four participants), mercaptopurine (four participants), amitriptyline (one participant), rectal hydrocortisone suppository (3 mg, one participant), and lansoprazole (one participant). One participant had a history of treatment with prednisone (10 mg) during the month the study was performed. Crohn disease severity was assessed by using the previously validated Pediatric Crohn's Disease Activity Index (PCDAI), with PCDAI scores categorized as follows: quiescent disease (0–10), mild disease activity (11–30), moderate to severe disease activity (>30) (16). The mean PCDAI was 8 ± 9 [standard deviation (SD)] and range was 0–25, indicating quiescent to mild disease activity in all subjects at the time of evaluation. All subjects had moderate to severe disease activity at the time of diagnosis of Crohn disease.
Study protocol.
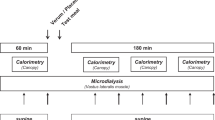
To determine the effect of enteral nutrition on protein turnover, Leu and Phe kinetics were measured using stable isotopes under fasted and fed conditions during a single study visit. Figure 1 shows the overall study design. Subjects came to the General Clinical Research Center on the morning of the study, after an 8-h overnight fast. Body composition (total body lean and fat mass) was measured by dual-energy x-ray absorptiometry using a Lunar DPXL instrument (Lunar, Madison, WI). The inpatient study consisted of two parts over 8 h: a basal fasted period and a period during which subjects were consuming enteral nutrition. Two i.v. catheters were inserted, one in an antecubital vein for administration of stable isotope tracers and the second in a dorsal contralateral hand vein for sampling of arterialized blood. After a baseline blood sample was obtained, a primed continuous infusion of [d3]Leu (actual infusion rate 3.5 μmol/kg/h), [d5]Phe (actual infusion rate 1.25 μmol/kg/h), and [d2]tyrosine (actual infusion rate 0.7 μmol/kg/h) in normal saline was started and continued for the remainder of the study. In addition, [d4]tyrosine (0.44 μmol/kg) was given (prime only) to calculate rates of Phe hydroxylation. All isotopes were pyrogen tested before use in the studies (Table 2).
The basal fasted study period lasted 3.5 h, with blood samples obtained at 60, 120, 150, 180, and 210 min. The plasma was immediately separated and frozen at −70° for later analysis. After the basal fasted study period, 5 oz of an enteral nutrition formula (Boost) with a priming dose of 1-[13C]Leu (5 μmol/kg) and [13C]Phe (2.5 μmol/kg) was taken orally. Hourly oral feedings of enteral nutrition formula with 1-[13C]Leu (5 μmol/kg/h) and [13C]Phe (2.5 μmol/kg/h) were administered for a period of 5 h. The continuous i.v. tracer infusion was maintained during this period. Blood samples were obtained at 240, 300, 360, 420, 450, and 480 min, and the plasma was frozen for later analysis. The syringes containing the stable isotopes were weighed before and after infusion to determine the volume of tracer solution actually delivered.
Analytic methods.
Plasma enrichments of Leu, α-ketoisocaproic acid (KIC, the intracellular transamination product of Leu), Phe, and tyrosine were determined by electron impact ionization and selected ion monitoring on a gas chromatograph-mass spectrometer (GCMS) (model 5970, Hewlett-Packard, Palo Alto, CA). The enrichments of plasma Leu, Phe, and tyrosine were determined by monitoring ions 302 and 303 (1-[13C]Leu), 302 and 305 ([d3]Leu), 336 and 337 ([13C]Phe), 234 and 239 ([d5]Phe), 466 and 468 ([d2]tyrosine), and 466 and 470 ([d4]tyrosine) after derivatization to the tertiary butyldimethylsilyl derivatives (17,18). The plasma enrichment of KIC was determined after derivatization to the O-trimethylsilylquinoxalinol by monitoring ions 232 and 233 ([13C]KIC), and 259 and 262 ([d3]KIC) (19). The final value for all determinations was corrected using an enrichment calibration curve. When multiple isotopes of an amino acid were used, the increase in the background enrichment of the higher mass isotope produced by the lower mass isotope was accounted for using a correction curve. The mean enrichment values of four samples taken during the enrichment plateau (steady state) of the fasted study period and three samples taken during steady state of the fed study period were used for calculations in each subject. Steady state was achieved for all subjects as defined by the absence of significant slope in plasma enrichment by linear regression analysis.
Calculations.
Plasma enrichments of Phe were used to calculate the Ra of Phe. To calculate the Ra of Leu, plasma enrichments of KIC were used because this has been shown to closely approximate intracellular Leu enrichment, thereby providing a more accurate assessment of whole-body proteolysis (20). The total Ra of Leu, Phe, and tyrosine were calculated by measuring tracer dilution at steady state as modified for stable isotopic tracers [Ra = (100 ÷ EP − 1) × I]; where EP is the steady state enrichment of the specific isotope in the plasma and I is the rate of tracer infusion (21). Because Leu and Phe are both essential amino acids, their Ra during the fasted state reflects whole-body proteolysis (22). During the fed state, endogenous Ra of Leu and Phe (reflecting proteolysis) were calculated by subtracting the rate of exogenous administration of unlabeled Leu or Phe (derived from nutritional content of the enteral formula) from their measured total Ra (21). Endogenous Ra of Leu was calculated using the rate of exogenous administration of unlabeled Leu and the total Ra of Leu based on both KIC enrichment and Leu enrichment. In this study, the unlabeled Leu intake was 89 ± 14 μmol/kg/h and the unlabeled Phe intake was 32 ± 5 μmol/kg/h.
The intake of Leu and Phe in the enteral feeding was adjusted for first pass splanchnic uptake (23). The splanchnic uptake of Leu was calculated as follows: splanchnic extraction Leu (%) = [1 − (total Ra of Leu from i.v. tracer ÷ total Ra of Leu from enteral tracer)] × 100. The splanchnic extraction of Phe (%) was calculated in an identical manner. The absolute amount of Leu or Phe extracted by the splanchnic bed was calculated by multiplying the percent extraction by the enteral intake of Leu or Phe.
Rates of Phe hydroxylation to tyrosine were calculated as described previously (18,21). Phe utilization for protein synthesis was calculated by subtracting the rate of Phe hydroxylation to tyrosine from the total Ra of Phe, as Phe is presumed to be irreversibly lost either by its degradation pathway via its conversion into tyrosine or by incorporation into protein (18,21).
Statistics.
Paired t tests were used to compare measures of proteolysis and protein synthesis under fasted and fed conditions. Data are presented as mean ± SD. P values of <0.05 were considered statistically significant.
RESULTS
Isotopic steady state was achieved for all subjects during the two study periods (Fig. 2). The endogenous Ra values for Leu (calculated from Leu enrichments) and Phe, reflecting release of Leu and Phe into the circulation due to proteolysis in the fasted and fed state are shown in Figure 3. In response to enteral nutrition (mean protein intake 0.12 ± 0.03 g/kg/h), the endogenous Ra of Leu and Phe decreased 40%. The mean endogenous Ra values for Leu calculated from the KIC enrichments were 137 ± 6 μg/kg/h (fasted) and 96 ± 22 (fed), reflecting a decrease of 30% in response to enteral nutrition, p < 0.05.
The rate of Phe hydroxylation, reflecting irreversible Phe loss, did not change significantly during the study (Fig. 3). The mean values for the rate of splanchnic uptake of Leu and Phe were 31 ± 10 and 4 ± 3 μmol/kg/h, respectively. Expressed as a percentage of Leu and Phe intake, splanchnic uptake of Leu was 35 ± 10% and of Phe was 13 ± 12%. In response to enteral nutrition, utilization of Phe for protein synthesis increased by 25% (Fig. 3).
DISCUSSION
In this study, we evaluated the effect of acute enteral nutrition on splanchnic uptake of Leu and Phe, whole-body proteolysis, Phe catabolism, and protein synthesis in adolescents with Crohn disease during a period of relatively good health. Our results demonstrate that in clinically stable adolescents with inactive Crohn disease, enteral nutrition promotes anabolism acutely by both suppressing proteolysis and increasing protein synthesis. During feeding, the rate of utilization of Phe for protein synthesis exceeded the endogenous Ra of Phe, reflecting a condition that permits protein accretion.
Previous studies of healthy adults and prepubertal children have shown that enteral feeding is associated with a suppression of whole-body proteolysis, with little change in rates of protein synthesis (4–7,24). Rates of acute suppression of proteolysis in response to enteral feeding in this study were similar to those reported previously in healthy children evaluated using the same methodology (7). This previous study by Kien et al. (7) used the same experimental design, assessing the response to enteral feeding with Ensure with stable isotopes of Leu, although it did not account for splanchnic uptake of Leu. In the current study, the response to feeding was assessed with two essential amino acids (Leu and Phe). Consistent results were achieved despite the very different metabolic pathways of the two amino acids, supporting the validity of our results.
We also found that the rates of utilization of Phe for protein synthesis increased significantly by 25% with feeding. This is similar to the increase in protein synthesis with enteral feeding previously reported for prepubertal subjects with cystic fibrosis (7) and for newborn infants (25). These results suggest that adolescents with quiescent Crohn disease accomplish protein accretion by increases in protein synthesis and suppression of proteolysis rather than suppression of proteolysis alone.
Numerous studies in adults have demonstrated that enteral feeding induces suppression of proteolysis and promotion of lean body mass accrual through induction of anabolic hormonal processes (26–31). However, few studies have evaluated the effect of enteral feeding on proteolysis and protein synthesis in adults or children with inactive or active Crohn disease. Bourreille et al. (8) evaluated glutamine utilization in six adult patients with Crohn disease in remission with near-normal nutritional status, before and after nasogastric administration of [1-13C]glutamine. The Bourreille et al. study demonstrated similar rates of proteolysis, glutamine oxidation, and splanchnic uptake of glutamine in response to administration of enteral tracer among adult subjects with Crohn disease in remission compared with healthy subjects (8). Thus, it is logical to presume that protein turnover in children with inactive Crohn disease probably reflects that of healthy children, analogous to the adult study.
Studies to examine the balance between proteolysis and protein synthesis in response to nutrition in pediatric patients with Crohn disease are scarce (3,9,10). Thomas et al. (10) evaluated whole-body protein metabolism, in the fasted state, in 10 children with active Crohn disease who were treated either with an elemental diet or a usual diet plus prednisolone and sulfasalazine. Although rates of proteolysis and protein synthesis decreased among both treatment groups, there were no differences between the treatment groups in overall protein balance before and after treatment. Although the study did not report an improvement in protein balance during remission of Crohn disease, this study was performed only in the fasted state and thus did not include data on protein balance in the fed state. Motil et al. (3,9) evaluated whole-body protein metabolism in the fed state, after consumption of a usual diet and after consumption of a diet supplemented with overnight feeding for 7 mo in six adolescents with Crohn disease and growth failure. After long-term nutritional supplementation, subjects had significant increases in Leu incorporation into body protein and decreased rates of Leu oxidation. The differences in methodology of the studies, one performed in the fed state and the other in the fasting state, may explain the discrepancy in the findings of the studies. There is a lack of studies evaluating protein turnover under fasted and fed conditions.
Previous studies in pediatrics have not evaluated protein turnover in the fed and fasted states while accounting for splanchnic uptake of diet-derived amino acids. This is important as the estimation of rates of protein breakdown and synthesis in the fed state utilizing tracer studies depends on knowledge of the percentage of diet-derived amino acids removed on first pass by the intestine and/or liver. In this study, splanchnic uptake of both Leu and Phe was determined in the fed state to estimate rates of protein breakdown, oxidation, and synthesis accurately. A previous study measured splanchnic uptake of Leu in five healthy children in the fed state using very similar methodology as the current study (23). Results of this study showed healthy children to have a splanchnic uptake of Leu of 24 ± 25.6%. Our results suggest that splanchnic uptake of Leu may have been higher in our study participants (35 ± 10%). We are not aware of other studies evaluating the splanchnic uptake of Phe in children. Previous studies in adults have shown the percentage of splanchnic uptake of Phe to be greater than that of Leu (32–34). This was not the case in this study, and the metabolic significance of this is uncertain as previous studies in pediatrics have not evaluated splanchnic uptake of both amino acids.
The limitations of this study include a lack of a control group. Although comparisons with healthy adults and children were made relative to values in the literature, the inclusion of a control group of healthy children for comparison would have strengthened this study. Metabolic research such as this in healthy pediatric volunteers is difficult to perform and is extremely limited. The selection of participants with quiescent Crohn disease may also be viewed as a limitation. Our data do not provide evidence that these findings are specifically related to Crohn disease, but may reflect that of healthy age-matched children. Results would likely be different in adolescents with active disease. Still, the current study extends the knowledge base in the field, providing a basis for further study in patients with more severe disease. Finally, the acute effects of enteral nutrition were analyzed rather than the effects of a prolonged course of enteral nutrition as a treatment. Although the present study was not designed to evaluate the effect of long-term enteral nutrition, future research should address this. Despite these limitations, the current study provides unique information on which to build further research in a field where there is a lack of data in pediatrics.
If during this study absorbed protein intake were overestimated, then estimates of proteolysis in the fed state would be underestimated. This error is not likely to have occurred, as the participants in this study had mild disease activity and in normal humans, >99% of ingested protein is absorbed in the gastrointestinal tract (35). Moreover, amino acids tracers are unlikely to be malabsorbed. In calculating Phe utilization for protein synthesis, it is presumed that Phe is irreversibly lost either by its degradation pathway via its conversion into tyrosine or by incorporation into protein (18,21). In theory, Phe may not be irreversibly lost by incorporation into protein because it may be used for the synthesis of proteins with rapid turnover such as intestinal enzymes that could subsequently be broken down and reabsorbed from the intestinal lumen within the time course of the study. Still, it is likely that in the time frame of the study little recycling of Phe may have occurred.
In summary, in clinically stable adolescents with inactive Crohn disease, acute administration of enteral nutrition promotes anabolism by suppressing proteolysis and increasing protein synthesis. Rates of suppression of proteolysis were similar to those reported previously in healthy children. With this knowledge, future research can be directed toward evaluation of children with more severe disease.
Abbreviations
- KIC:
-
α-ketoisocaproic acid
- Leu:
-
leucine
- Phe:
-
phenylalanine
- Ra:
-
rate of appearance
References
Azcue M, Rashid M, Griffiths A, Pencharz PB 1997 Energy expenditure and body composition in children with Crohn's disease: effect of enteral nutrition and treatment with prednisolone. Gut 41: 203–208
Bannerjee K, Camacho-Hubner C, Babinska K, Dryhurst KM, Edwards R, Savage MO, Sanderson IR, Croft NM 2004 Anti-inflammatory and growth-stimulating effects precede nutritional restitution during enteral feeding in Crohn disease. J Pediatr Gastroenterol Nutr 38: 270–275
Motil KJ, Grand RJ, Maletskos CJ, Young VR 1982 The effect of disease, drug, and diet on whole body protein metabolism in adolescents with Crohn disease and growth failure. J Pediatr 101: 345–351
Melville S, McNurlan MA, McHardy KC, Broom J, Milne E, Calder AG, Garlick PJ 1989 The role of degradation in the acute control of protein balance in adult man: failure of feeding to stimulate protein synthesis as assessed by L-[1-13C]Leu infusion. Metabolism 38: 248–255
Motil KJ, Bier DM, Matthews DE, Burke JF, Young VR 1981 Whole body leucine and lysine metabolism studied with [1-13C]leucine and [alpha-15N]lysine: response in healthy young men given excess energy intake. Metabolism 30: 783–791
Welle S, Thornton C, Statt M, McHenry B 1994 Postprandial myofibrillar and whole body protein synthesis in young and old human subjects. Am J Physiol 267: E599–E604
Kien CL, Zipf WB, Horswill CA, Denne SC, McCoy KS, O'Dorisio TM 1996 Effects of feeding on protein turnover in healthy children and in children with cystic fibrosis. Am J Clin Nutr 64: 608–614
Bourreille A, Humbert B, Maugere P, Galmiche JP, Darmaun D 2004 Glutamine metabolism in Crohn's disease: a stable isotope study. Clin Nutr 23: 1167–1175
Motil KJ, Grand RJ, Matthews DE, Bier DM, Maletskos CJ, Young VR 1982 Whole body leucine metabolism in adolescents with Crohn's disease and growth failure during nutritional supplementation. Gastroenterology 82: 1359–1368
Thomas AG, Miller V, Taylor F, Maycock P, Scrimgeour CM, Rennie MJ 1992 Whole body protein turnover in childhood Crohn's disease. Gut 33: 675–677
Beattie RM, Camacho-Hubner C, Wacharasindhu S, Cotterill AM, Walker-Smith JA, Savage MO 1998 Responsiveness of IGF-I and IGFBP-3 to therapeutic intervention in children and adolescents with Crohn's disease. Clin Endocrinol (Oxf) 49: 483–489
Savage MO, Beattie RM, Camacho-Hubner C, Walker-Smith JA, Sanderson IR 1999 Growth in Crohn's disease. Acta Paediatr Suppl 88: 89–92
Street ME, de'Angelis G, Camacho-Hubner C, Giovannelli G, Ziveri MA, Bacchini PL, Bernasconi S, Sansebastiano G, Savage MO 2004 Relationships between serum IGF-1, IGFBP-2, interleukin-1beta and interleukin-6 in inflammatory bowel disease. Horm Res 61: 159–164
Zoli G, Katelaris PH, Garrow J, Gasbarrini G, Farthing MJ 1996 Increased energy expenditure in growing adolescents with Crohn's disease. Dig Dis Sci 41: 1754–1759
Burnham JM, Shults J, Semeao E, Foster BJ, Zemel BS, Stallings VA, Leonard MB 2005 Body-composition alterations consistent with cachexia in children and young adults with Crohn disease. Am J Clin Nutr 82: 413–420
Hyams JS, Ferry GD, Mandel FS, Gryboski JD, Kibort PM, Kirschner BS, Griffiths AM, Katz AJ, Grand RJ, Boyle JT, et al. 1991 Development and validation of a pediatric Crohn's disease activity index. J Pediatr Gastroenterol Nutr 12: 439–447
Schwenk WF, Berg PJ, Beaufrere B, Miles JM, Haymond MW 1984 Use of butyldimethylsilylation in the gas chromatographic/mass spectrometric analysis of physiologic compounds found in plasma using electron-impact ionization. Anal Biochem 141: 101–109
Thompson GN, Pacy PJ, Merritt H, Ford GC, Read MA, Cheng KN, Halliday D 1989 Rapid measurement of whole body and forearm protein turnover using a [2H5]Phe model. Am J Physiol 256: E631–E639
Wolfe RR 1992 Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetic Analysis. Wiley-Liss, New York, p 471
Matthews DE, Schwarz HP, Yang RD, Motil KJ, Young VR, Bier DM 1982 Relationship of plasma leucine and alpha-ketoisocaproate during a L-[1-13C]leucine infusion in man: a method for measuring human intracellular leucine tracer enrichment. Metabolism 31: 1105–1112
Denne SC, Karn CA, Ahlrichs JA, Dorotheo AR, Wang J, Liechty EA 1996 Proteolysis and phenylalanine hydroxylation in response to parenteral nutrition in extremely premature and normal newborns. J Clin Invest 97: 746–754
Denne SC, Kalhan SC 1987 Leucine metabolism in human newborns. Am J Physiol 253: E608–E615
Kien CL, Horswill CA, Zipf WB, McCoy KS, Denne SC 1999 Splanchnic uptake of leucine in healthy children and in children with cystic fibrosis. Pediatr Res 45: 680–683
Denne SC, Karn CA, Liechty EA 1992 Leucine kinetics after a brief fast and in response to feeding in premature infants. Am J Clin Nutr 56: 899–904
Denne SC, Rossi EM, Kalhan SC 1991 Leucine kinetics during feeding in normal newborns. Pediatr Res 30: 23–27
Biolo G, Inchiostro S, Tiengo A, Tessari P 1995 Regulation of postprandial whole-body proteolysis in insulin-deprived IDDM. Diabetes 44: 203–209
Denne SC, Liechty EA, Liu YM, Brechtel G, Baron AD 1991 Proteolysis in skeletal muscle and whole body in response to euglycemic hyperinsulinemia in normal adults. Am J Physiol 261: E809–E814
Fukagawa NK, Minaker KL, Rowe JW, Goodman MN, Matthews DE, Bier DM, Young VR 1985 Insulin-mediated reduction of whole body protein breakdown. Dose-response effects on leucine metabolism in postabsorptive men. J Clin Invest 76: 2306–2311
Tessari P, Trevisan R, Inchiostro S, Biolo G, Nosadini R, De Kreutzenberg SV, Duner E, Tiengo A, Crepaldi G 1986 Dose-response curves of effects of insulin on leucine kinetics in humans. Am J Physiol 251: E334–E342
Castellino P, Luzi L, Simonson DC, Haymond M, DeFronzo RA 1987 Effect of insulin and plasma amino acid concentrations on leucine metabolism in man. Role of substrate availability on estimates of whole body protein synthesis. J Clin Invest 80: 1784–1793
Giordano M, Castellino P, DeFronzo RA 1996 Differential responsiveness of protein synthesis and degradation to amino acid availability in humans. Diabetes 45: 393–399
Biolo G, Tessari P, Inchiostro S, Bruttomesso D, Fongher C, Sabadin L, Fratton MG, Valerio A, Tiengo A 1992 Leucine and phenylalanine kinetics during mixed meal ingestion: a multiple tracer approach. Am J Physiol 262: E455–E463
Castillo L, Chapman TE, Yu YM, Ajami A, Burke JF, Young VR 1993 Dietary arginine uptake by the splanchnic region in adult humans. Am J Physiol 265: E532–E539
Matthews DE, Marano MA, Campbell RG 1993 Splanchnic bed utilization of leucine and phenylalanine in humans. Am J Physiol 264: E109–E118
Groff JL, Gropper SS 2000 Advanced nutrition and human metabolism. Wadsworth/Thomson Learning, Belmont, CA, pp 163–219
Acknowledgements
These studies would not have been possible without the assistance of the nurses and staff of the General Clinical Research Center and the commitment of the study volunteers and their parents.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the Clarian Values Fund for Research, the Human Growth Foundation, and U.S. Public Health Service grants K23 RR17250-01, HD 29153, and M01-RR 00750 through the General Clinical Research Center at Indiana University School of Medicine.
Rights and permissions
About this article
Cite this article
Hannon, T., Dimeglio, L., Pfefferkorn, M. et al. Acute Effects of Enteral Nutrition on Protein Turnover in Adolescents with Crohn Disease. Pediatr Res 61, 356–360 (2007). https://doi.org/10.1203/pdr.0b013e318030d11c
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/pdr.0b013e318030d11c