Abstract
Growth, bone, and body composition were studied at prepuberty in former very low birth weight (VLBW) infants who received dexamethasone (DEX) for bronchopulmonary dysplasia (BPD) compared with VLBW infants without DEX and term-born infants (TERM) to identify early life risk factors for later low bone mass. Children (56 girls/63 boys, 5–10 y) previously studied in neonatal life were recruited into three groups: VLBW + DEX, VLBW – DEX, and TERM children. Anthropometry and whole body bone, fat, and lean mass were measured. At prepuberty, the average height and weight for VLBW + DEX group were significantly lower than that for VLBW – DEX and TERM. Both VLBW groups had lower bone mass even adjusted for height and lean mass than TERM children and lower lean mass both total and adjusted for height. Z-scores for whole body bone mineral content below –1.5 occurred in 27.9% of VLBW + DEX children. The key factors for low bone mass were earlier gestational age and having BPD with DEX in neonatal life. In former VLBW infants, growth and bone mass attainment before puberty can be predicted from early life variables. VLBW + DEX children may be protected from overweight, but are at risk for short stature and low bone mass.
Similar content being viewed by others
Main
VLBW infants often develop BPD and, in the early 1990s, DEX was frequently prescribed to improve pulmonary compliance and facilitate earlier weaning from the ventilator. As a glucocorticoid, DEX alters skeletal metabolism (1,2), induces protein catabolism, decreases lean mass (3), and increases fat accumulation sometimes leading to obesity (4). DEX therapy is associated with growth restriction in infants (5). Preterm BPD infants who were treated with DEX demonstrated impaired growth, with a lower whole body fat and lean mass than healthy term infants during the first year of life (6). Compared with nonsteroid-treated VLBW control infants, the VLBW infants treated with DEX for BPD also showed lower weight and shrinkage of the lower leg length (7). In our previous studies in both VLBW infants (8,9) and infant piglets (1,10), growth in weight, length, and bone mass was compromised by DEX administration in early life, although in the human infants the effects of BPD and ventilatory history may also contribute.
The long-term consequences of exposure to exogenous steroid drugs in infants and children are just beginning to be evaluated. In a 3-y follow-up study (11), physical growth outcomes were not impaired in neonates who received tapering doses of DEX for 7 d. However, in children with acute lymphoblastic leukemia (ALL), DEX therapy was associated with suppressed short-term linear growth and bone turnover (12). The long-term sequences of steroid therapy in survivors of ALL sometimes manifest as reduced growth and bone mass (13), yet such findings are not consistent (14).
We hypothesized that in our study population both VLBW and DEX therapy in early life present risk factors for restricted growth, altered body composition and delayed bone mineralization in later life. The present study was designed to assess status of growth and body composition in former VLBW infants who were treated with DEX for BPD and who are currently at prepubertal age. Growth attainment and bone, fat and lean mass were compared between children who were born prematurely and with VLBW but were or were not treated with DEX, and with children who were born at term and appropriate for gestational age.
METHODS
Subjects.
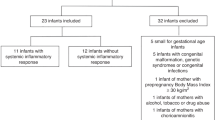
Subjects were recruited by letters of invitation sent to parents of former premature and term infants (most all were Caucasian) who participated in nutrition trials, predominantly of formula feeding, conducted in our laboratory when they were infants at McMaster Children's Hospital in Hamilton and were followed to 1 y corrected age. In total, 339 letters were sent and 140 replied (135 consented/5 denied). There were 65 letters returned as “address unknown” and 134 letters received no response. Finally, one hundred and nineteen children (56 girls and 63 boys, 5-10 y) were agreed to participate in the single visit appointment. The subjects were allocated to one of three groups: VLBW + DEX group (n = 47) were children who had birth weight <1500 g and were treated with at least one full course (7 d) of DEX for BPD during the neonatal period; VLBW-DEX group (n = 36) were children who were born preterm and also had birth weight <1500 g, but without BPD and did not receive DEX; and the TERM group (n = 36) were normal healthy children who are of similar age and gender as the two preterm groups but who were born at term and with birth size appropriate for gestational age. For the VLBW + DEX group, DEX was prescribed as a tapered dosing regimen, as detailed in the original publications (8,9,15) with mean cumulative doses ranging from 6 to 8 mg. This study was reviewed and approved by the Research Ethics Board of McMaster University and informed consent was obtained from parents of all subjects.
Growth measurements.
The subjects' body weight and height were measured wearing clothing, but without shoes using a standard medical stadiometer. The results were plotted on the most recent reference growth standards from the Center for Disease Control and Prevention (16), from which the values of height-for-age percentile and weight-for-age percentile were obtained. The Z-scores of height and weight were calculated with ANTHRO software (Version 1.02, CDC/WHO). From the anthropometric measurements, body mass index (BMI) was calculated. Tanner stage of all subjects was self-assessed according to validated self-administered tool with photographs (17). To confirm the accuracy of the self-administered method, Tanner stage was reassessed by a pediatric endocrinologist (J.V.) in 20 of the subjects and the physician determination of Tanner stage was consistent with the results of self-assessment. The height of biologic parents was obtained by self-report.
Analysis of calcium intake and physical activity.
Information regarding calcium intake and physical activity status was obtained by questionnaires. Daily calcium (Ca) intake from foods and supplements was evaluated by a quick quantity method based on a validated food frequency questionnaire reflecting 1-y diet (18). The method of activity assessment was adapted from Hay and Cairney (19), in which activity score was calculated based on a scale of –100 (100% inactive) to +100 (100% active).
Body composition and bone mass measurements.
Bone mass of the 1st–4th lumbar vertebrae and whole body bone, fat, and lean mass were assessed using DXA (Hologic QDR1000W, Waltham, MA). The software used was V4.76P for spine analysis and V5.73 for whole body analysis. Five children did not complete the DXA as they were unable to lie still during the scan. Quality control for the DXA machine was performed using a phantom spine. For practical and ethical reasons, all DXA measurements in children were a single scan. WBBMC Z-score was calculated using reference values for children from the web database ( www.bcm.tmc.edu/bodycomlab/ ) (20) and the absolute values were adjusted for lean mass, height, and bone area and to remove the BMC attributed to the head region. As suggested by Wells and Cole (21), fat mass index [FMI = fat mass (kg)/height (cm) (2)] and fat free (lean) mass index [FFMI = lean mass (kg)/height (cm) (2)] were calculated. Also, percentages of fat, lean, and WBBMC were calculated as a function of total mass of the specific compartment divided by total body weight. Values for bone and body composition from birth to 1 y corrected age were obtained from our research records of DXA scans with the same instrument in our laboratory during the nutrition studies in early life. A standard questionnaire was used to obtain information on bone fracture during childhood and use of steroid drugs after early neonatal life. Cumulative steroid dose in infancy was recorded from neonatal charts when the infants were enrolled in the nutrition studies in the first year life.
Statistical analysis.
For each outcome measurement, mean and SD were calculated. t Test, one-way ANOVA, analysis of covariance (ANCOVA) using age as a covariate, and χ2 analysis were used to compare the difference in the outcomes between the three groups. Post hoc analysis was performed using Bonferroni multiple comparison test. Simple linear regression analysis was performed to investigate the relationship between current outcomes with growth, body composition, and bone mass in early infancy. To evaluate factors predictive of bone mass at prepuberty, stepwise regression analysis was conducted with current WBBMC Z-score as the dependent variable. All analyses were done using Prism (GraphPad Software, San Diego, CA), NCSS 2000 (NCSS Statistical Software, Kaysville, VT), and SPSS 11.0 (SPSS Inc., Chicago, IL) software and differences were considered significant if p < 0.05.
RESULTS
There were no significant differences in the mean ages between the VLBW + DEX group and TERM group, but the VLBW + DEX group was 1.1 y older than VLBW – DEX group (Table 1). Based on the self-evaluation, there were 12 girls (7 in the VLBW + DEX group, 1 in the VLBW – DEX group, and 4 in the TERM group) with Tanner Stage >1 (girls rated their breast and pubic hair development using schematics of 5 Tanner stage) and there were 4 boys (3 in the TERM group and 1 in the VLBW + DEX group) with Tanner stage >1. The male/female ratio was balanced within the three groups. The three groups had a similar calcium intake and the heights of biologic parents were similar (Table 1). Significantly lower activity was observed for the VLBW + DEX children compared with the VLBW – DEX children (Table 1). Hairline fractures occurred in 4 children (2 in VLBW + DEX group and 2 in TERM group). Occasional use of inhaled steroids occurred in 34 children (40% in the VLBW + DEX group, 28% in the VLBW – DEX group and 14% in the TERM group; p = 0.0013).
Height.
As a group, height for the VLBW + DEX children was 9.0 cm shorter than TERM children (Table 1). Mean height Z-score of the VLBW + DEX children was significantly lower than that of both VLBW – DEX and TERM children (Figure 1a). The VLBW – DEX children also had significantly lower height Z-score than TERM children. The incidence of short stature (defined as height-for-age <10th percentile) for the VLBW + DEX group was 19.1%, which was significantly higher than both the VLBW – DEX group at 2.8% and the TERM group at 5.6% (Table 1).
Weight and BMI.
The mean weight for the VLBW + DEX group was 7.0 kg lighter than the TERM group (Table 1). Mean weight Z-score of the VLBW + DEX group was significantly lower than that of both VLBW – DEX and TERM groups (Fig. 1b). Also, the VLBW – DEX group had significantly lower weight Z-score than the TERM group. BMI was similar between the two VLBW groups. However, only VLBW + DEX group had a significantly lower BMI than the TERM group (Table 1).
Relationship of body weight and height between prepuberty and early infancy. According to the available growth records of these children during neonatal life, the VLBW + DEX group demonstrated significantly lower birth weight (BW) and younger gestational age than the VLBW – DEX and TERM children (Table 1). For the combined VLBW groups, height Z-score at prepuberty was associated with body length at term and 1 y corrected age (Fig. 2, a and b), and body weight at 1 y corrected age was predictive of body weight Z-score at prepuberty (Fig. 3).
Fat and lean mass at prepuberty.
Using age as a covariate, the VLBW + DEX group had lower mean fat and lean mass than the TERM group both in absolute mass and as FMI and FFMI, while there was no difference between the two VLBW groups (Table 2). Compared with the TERM group, the VLBW – DEX group had lower FFMI, but not FMI (Table 2). There were no differences in FMI and FFMI between boys and girls at prepuberty. Fat and lean mass as a percentage of total body weight were similar between the three groups. Compared with the normal range for percentage body fat for different ages of children (21), 19% of children from VLBW + DEX group were overweight, 31% of the VLBW – DEX and 40% of the TERM children were overweight (VLBW + DEX versus TERM group, p = 0.04). Only three boys in total from the VLBW groups were classified as obese.
Relationship of fat and lean mass between prepuberty and early infancy.
Based on our previously available records on fat and lean mass for the combined former VLBW preterm children, at term corrected age, the VLBW + DEX and VLBW – DEX infants had similar FMI and FFMI (Table 3). At 1 y corrected age, the VLBW + DEX infants had lower FMI but not FFMI compared with VLBW – DEX infants. At prepuberty, FMI did not correlate with measures at term or 1 y corrected age. However, there was a significant correlation in FFMI between 1 y corrected age and prepuberty (p < 0.0001, r = 0.66, n = 31).
Whole body and lumbar spine bone mass at prepuberty.
Bone mineral content and bone area in both whole body and lumbar spine were similar between the VLBW groups but significantly lower than TERM children (Table 2). After adjustment of WBBMC, for height, for bone area and after subtracting the BMC of the head region with or without adjustment for lean mass (Table 2), lower bone mass for both former VLBW groups compared with TERM children was still evident. For WBBMC/lean, the lower value in VLBW without DEX did not reach statistical significance (Table 2). The z-scores for WBBMC in both VLBW groups were also lower compared with TERM groups (Fig. 4). The incidence of low bone mass (WBBMC Z score <–1.5) was greater in VLBW + DEX children (27.9%) than the VLBW – DEX (19.4%) or TERM children (8.6%).
Relationship of bone mass between prepuberty and early infancy.
Because infant WBBMC Z-scores cannot be calculated from the pediatric database (age limitation as 2–22 y old) (20), we did the bone mass correlation with WBBMC adjusted for total body weight. The subjects' WBBMC at term corrected age was associated with the WBBMC at prepuberty (Fig. 5).
Predictive factors of WBBMC Z-score for former preterm infants at prepubertal age.
Among factors at the time of study (body height, weight, calcium intake, physical activity), at early life (birth weight, gestational age, having BPD with DEX treatment, WBBMC values at term and 1 y corrected age), and genetic related (parents height), we found that earlier gestational age was the main contributor to low bone mass for boys at prepuberty (Table 4). For girls, current height Z-score, having BPD with DEX treatment in early infancy, WBBMC at 1 y corrected age and mother's height were significantly associated with WBBMC Z-score at prepuberty (Table 4). Stepwise regression showed that current height Z-score was the significant predictor of low WBBMC Z-score for girls at prepuberty (Table 5). Mother's height and having BPD with DEX treatment were the two predictive factors for height Z-score of girls (data not shown).
DISCUSSION
Growth.
At prepubertal age, the children who were the smallest former VLBW infants and who had severely compromised lung function and required therapy with steroid drugs in early neonatal life were still shorter and lighter in physical growth than term born children and age and gender-matched former preterm VLBW infants who had not received DEX. Depending on gender, predictive factors identified as contributors to the observed growth failure included extreme low birth weight, a residual effect of intervention with DEX for 7 d or more in early life as was clinical practice at the time (8,9,15), and/or a lesser nutritional and general health status due to extreme prematurity. Because it is unlikely to find a true VLBW comparison group of children with similar severity of lung disease but without DEX treatment, the etiology of the growth failure cannot be fully attributed to neonatal exposure to DEX. However, our study provides evidence that BPD and treatment with DEX in early life in VLBW infants are markers for particular risk of sub-optimal growth and bone mass before puberty.
Significant correlations between birth size and growth in early life with growth attainment at prepuberty support the theory that growth in later life was programmed by intrauterine and neonatal life. Our follow-up measures of growth are consistent with previous observations of incomplete catch-up growth in preterm children compared with their term peers (23,24). Specific to VLBW premature infants (gestational age <29 wk) with or without BPD, Korhonen et al. (25) found both groups to be similarly shorter and lighter compared with term infants at 7 y of age but there were no differences in growth between the former BPD or non-BPD groups. If BPD per se does not have long-term consequences to height growth, then the observed significantly lower height percentile in the VLBW + DEX group with a BPD history may relate more to the neonatal DEX treatment or their extreme low birth weight. This supports our previous observation of compromised height growth in DEX treated VLBW infants at term and 6 mo corrected age (8).
Body composition.
Low birth weight may predict the risk of cardiovascular disease, hypertension, or diabetes (26,27) in adult life. As it is speculated that the relative proportion of fat to lean mass is an important predictor of these diseases, it is of interest to track development of body composition in VLBW infants. Our longitudinal measures of fat and lean mass in the two VLBW groups of children were conducted with the same DXA instrument at three time points: term corrected age, 1 y corrected age, and prepuberty. At term corrected age, there was a similar fat mass but significantly lower lean mass (p = 0.03) for the VLBW + DEX group compared with VLBW – DEX. The study by DeRegnier et al. (28) observed less arm fat and muscle accretion at 1 mo of age in VLBW infants with BPD. The difference perhaps relates to DEX therapy for BPD infants in our study but not in DeRegnier's study (28), because DEX administration may elevate serum leptin level, which regulates energy balance and correlates with body fat mass (29). However, by 1 y corrected age, lower fat mass in DEX-treated compared with nontreated infants was consistently observed in both studies.
The proposal for programming of lean body mass from intrauterine life based on birth weight (30) fits with our observations of lower FFMI in both VLBW groups at prepuberty compared with the TERM group. In slightly older (8–12 y) former preterm infants (<1850 g), a significantly lower FMI, but not FFMI was observed (31). The difference in findings may relate to the extremely low birth weight in our VLBW + DEX group. Such small size at birth may also manifest as subsequently reduced FMI in addition to FFMI. The long-term health implications of variation in body composition during childhood are as yet unproven but several theories have been postulated. Lower FMI at 8–12 y was proposed as protective against becoming overweight in later life (30). A lower FFMI may reflect a reduced metabolically active compartment with reduced glucose uptake and resulting insulin resistance (31). In contrast, Peralta-Carcelen et al. (32) reported that extremely LBW children had similar body composition compared with the term control children at adolescence after adjustment of body composition for weight. This illustrates the different methods used to interpret body composition could lead to different results. Clearly, longer-term follow-up of former preterm infants into young adulthood and beyond is essential before variation in body composition can be related to later health outcomes.
Bone accretion.
Reduced bone mass of preterm children was still evident after adjustment of WBBMC to remove the BMC attributed to the head, and when expressed as a function of height, lean mass, or bone area, a finding that contrasts with reports of formerly preterm infants having appropriate bone mass for their small body size at 12–20 y of age (33,34). The remarkable delay in bone mass accretion for the preterm children was attributable to their lung disease with steroid therapy in early life and/or their earlier gestational age, and possibly greater exposure to inhaled steroids in childhood. A strong positive correlation was also observed between growth in early infancy and bone accretion in childhood (35). The impact of the observed growth delays on achieving genetic potential for adult height and peak bone mass, or risk of adult-onset osteoporosis, is only speculative for this population. However, follow-up studies in former premature infants in peri-puberty (34,35), adolescence (33), and young adulthood (36) have consistently demonstrated height growth below expected (33–36) and low bone mass for age although proportional to height or lean mass (33–35). As none of the reported studies included children who had received DEX in neonatal life, follow-up of our cohort in young adulthood will be necessary. The sexual dimorphism observed in bone status at prepuberty may be related to genetic influence as it appeared that fathers' genetic factors had more impact on boys than girls. A differential effect of sex steroids (i.e. estrogen effects) on bone is not likely at this prepubertal age.
To achieve peak bone mass and attain catch-up growth in non-GH-deficient children of short stature, interest is emerging in the use of recombinant GH treatment. In children born small for gestational age (SGA), 3 y of GH treatment resulted in a normalization of height and a proportionate increase in bone maturation (37). In our previous study in growing piglets (38), GH therapy given concurrently with DEX partially attenuated DEX-induced body weight and length growth and low bone mineral content and bone density. The efficacy of GH therapy, either concomitant or subsequent to steroids, for infants and children treated with steroid drugs, will require future investigation.
In summary, many VLBW infants do not achieve optimal growth and body composition even after 6 to 9 y. With the emerging evidence that abnormal body composition may be indicative of altered metabolic pools and a marker for risk of morbidity in later life, continuous assessment of growth and body composition of former VLBW infants through to adult life is warranted.
Abbreviations
- BPD:
-
bronchopulmonary dysplasia
- DEX:
-
dexamethasone
- DXA:
-
dual energy X-ray absorptiometry
- VLBW:
-
very low birth weight
- WBBMC:
-
whole-body bone mineral content
References
Weiler HA, Wang Z, Atkinson SA 1995 Dexamethasone treatment impairs calcium regulation and reduces bone mineralization in infant pigs. Am J Clin Nutr 61: 805–811
Manolagas SC, Weinstein RS 1999 New developments in the pathogenesis and treatment of steroid-induced osteoporosis. J Bone Miner Res 14: 1061–1066
Weiler HA, Wang Z, Atkinson SA 1997 Whole body lean mass is altered by dexamethasone treatment through reductions in protein and energy utilization in piglets. Biol Neonate 71: 53–59
Van Dongen-Melman JE, Hokken-Koelega AC, Hahlen K, De Groot A, Tromp CG, Egeler RM 1995 Obesity after successful treatment of acute lymphoblastic leukemia in childhood. Pediatr Res 38: 86–90
Furman L, Hack M, Watts C, Borawski-Clark E, Baley J, Amini S, Hook B 1995 Twenty-month outcome in ventilator-dependent, very low birth weight infants born during the early years of dexamethasone therapy. J Pediatr 126: 434–440
Huysman WA, Ridder M, Bruin NC, Helmond G, Terpstra N, Goudoever JB, Sauer PJ 2003 Growth and body composition in preterm infants with bronchopulmonary dysplasia. Arch Dis Child Fetal Neonatal Ed 88: F46–F51
Crofton PM, Stirling HF, Schonau E, Ahmed SF 1996 Biochemical markers of bone turnover. Horm Res 45: 55–58
Weiler HA, Paes B, Shah JK, Atkinson SA 1997 Longitudinal assessment of growth and bone mineral accretion in prematurely born infants treated for chronic lung disease with dexamethasone. Early Hum Dev 47: 271–286
Ward WE, Atkinson SA, Donovan AM, Paes B 1999 Bone metabolism and circulating IGF-I and IGFBPs in dexamethasone-treated preterm infants. Early Hum Dev 56: 127–141
Guo CY, Ward WE, Cairns P, Atkinson SA 2000 Comparative response in growth and bone status to three dexamethasone treatment regimens in infants piglets. Pediatr Res 48: 238–243
Romagnoli C, Zecca E, Luciano R, Torrioli G, Tortorolo G 2002 Controlled trial of early dexamethasone treatment for the prevention of chronic lung disease in preterm infants: a 3-year follow-up. Pediatrics 109: e85
Ahmed SF, Tucker P, Mushtaq T, Wallace AM, Williams DM, Hughes IA 2002 Short-term effects on linear growth and bone turnover in children randomized to receive prednisolone or dexamethasone. Clin Endocrinol (Oxf) 57: 185–191
Mandel K, Atkinson S, Barr RD, Pencharz P 2004 Skeletal morbidity in childhood acute lymphoblastic leukemia. J Clin Oncol 22: 1215–1221
Van der Sluis IM, van den Heuvel-Eibrink MM, Hahlen K, Krenning EP, de Muinck Keizer-Schrama SM 2000 Bone mineral density, body composition, and height in long-term survivors of acute lymphoblastic leukemia in childhood. Med Pediatr Oncol 35: 415–420
Brunton JA, Saigal S, Atkinson SA 1998 Growth and body composition in infants with bronchopulmonary dysplasia up to 3 months corrected age: a randomized trial of a high-energy nutrient-enriched formula fed after hospital discharge. J Pediatr 133: 340–345
Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL 2000 CDC growth charts: United States. Adv Data Jun 8: 1–27
Morris NM, Udry JR 1980 Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc 9: 271–280
Musgrave KO, Giambalvo L, Leclerc HL, Cook RA, Rosen CJ 1989 Validation of a quantitative food frequency questionnaire for rapid assessment of dietary calcium intake. J Am Diet Assoc 89: 1484–1488
Hay J, Cairney J 2006 Development of the Habitual Activity Estimation Scale for clinical research—a systematic approach. Pediatr Exercise Sci 18: 193–202
Ellis KJ, Shypailo RJ, Hardin DS, Perez MD, Motil KJ, Wong WW, Abrams SA 2001 Z score prediction model for assessment of bone mineral content in pediatric diseases. J Bone Miner Res 16: 1658–1664
Wells JC, Cole TJ, ALSPAC study team 2002 Adjustment of fat-free mass and fat mass for height in children aged 8 y. Int J Obes Relat Metab Disord 26: 947–952
Taylor RW, Jones IE, Williams SM, Goulding A 2002 Body fat percentages measured by dual-energy X-ray absorptiometry corresponding to recently recommended body mass index cutoffs for overweight and obesity in children and adolescents aged 3-18 y. Am J Clin Nutr 76: 1416–1421
Powls A, Botting N, Cooke RW, Pilling D, Marlow N 1996 Growth impairment in very low birthweight children at 12 years: correlation with perinatal and outcome variables. Arch Dis Child Fetal Neonatal Ed 75: F152–F157
Niklasson A, Engsteom E, Hard AL, Wiklan KA, Hellstrom A 2003 Growth in very preterm children: a longitudinal study. Pediatr Res 54: 899–905
Korhonen P, Hyodynmaa E, Lenko HL, Tammela O 2004 Growth and adrenal androgen status at 7 years in very low birth weight survivors with and without bronchopulmonary dysplasia. Arch Dis Child 89: 320–324
Leeson CP, Taylor M, Morley R, Whincup PH, Lucas A, Deanfield JE 2001 Impact of low birthweight and cardiovascular risk factors on endothelial function in early adult life. Circulation 103: 1264–1268
Li C, Johnson MS, Goran MI 2001 Effects of low birth weight on insulin resistance syndrome in Caucasian and African-American children. Diabetes Care 24: 2035–2042
DeRegnier RA, Guilbert TW, Mills MM, Georgieff MK 1996 Growth failure and altered body composition are established by one month of age in infants with bronchopulmonary dysplasia. J Nutr 126: 168–175
Ng PC, Lam CW, Lee CH, Fok TF, Chan IH, Ma KC, Wong E 2002 Changes in serum leptin concentration after corticosteroid treatment in preterm infants. Acta Paediatr 91: 684–690
Singhal A, Wells J, Cole TJ, Fewtrell MS, Lucas A 2003 Programming of lean body mass: a link between birth weight, obesity, and cardiovascular disease?. Am J Clin Nutr 77: 726–730
Fewtrell MS, Lucas A, Cole TJ, Wells JC 2004 Prematurity and reduced body fatness at 8–12 y of age. Am J Clin Nutr 80: 436–440
Peralta-Carcelen M, Jackson DS, Goran MI, Royal SA, Mayo MS, Nelson KG 2000 Growth of adolescents who were born at extremely low birth weight without major disability. J Pediatr 136: 633–640
Weiler HA, Yuen CK, Seshia MM 2002 Growth and bone mineralization of young adults weighing less than 1500 g at birth. Early Hum Dev 67: 101–112
Fewtrell MS, Prentice A, Jones SC, Bishop NJ, Stirling D, Buffenstein R, Lunt M, Cole TJ, Lucas A 1999 Bone mineralization and turnover in preterm infants at 8–12 years of age: the effect of early diet. J Bone Miner Res 14: 810–820
Fewtrell MS, Prentice A, Cole TJ, Lucas A 2000 Effects of growth during infancy and childhood on bone mineralisation and turnover in preterm children aged 8–12 years. Acta Paediatr 89: 148–153
Saigal S, Stoskopf B, Streiner D, Paneth N, Pinelli J, Boyle M 2006 Growth trajectories of extremely low birth weight infants from birth to young adulthood: a longitudinal population-based study. Pediatr Res 60: 751–758
Arends NJ, Boonstra VH, Mulder PG, Odink RJ, Stokvis-Brantsma WH, Rongea-Westerlaken C, Mulder JC, Delemarre-Van de Waal H, Reeser HM, Jansen M, Waelkens JJ, Hokken-Koelega AC 2003 GH treatment and its effect on bone mineral density, bone maturation and growth in short children born small for gestational age: 3-year results of a randomized controlled GH trial. Clin Endocrinol (Oxf) 59: 779–787
Ward WE, Atkinson SA 1999 Growth hormone and insulin-like growth factor-I therapy promote protein deposition and growth in dexamethasone-treated piglets. J Pediatr Gastroenterol Nutr 28: 404–410
Acknowledgements
The authors thank research nurse Sue Steele for helping with scheduling of appointments and conduct of DXA scans and Janet Pinelli, Ph.D., for assistance with the models for regression analysis. The authors also thank the children and their parents for participating and cooperating in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by grants from the Hamilton Health Sciences Foundation and an operating fellowship from the Canadian Institutes for Health Research & Eli Lilly Co. Canada (DW).
Rights and permissions
About this article
Cite this article
Wang, D., Vandermeulen, J. & Atkinson, S. Early Life Factors Predict Abnormal Growth and Bone Accretion at Prepuberty in Former Premature Infants With/Without Neonatal Dexamethasone Exposure. Pediatr Res 61, 111–116 (2007). https://doi.org/10.1203/01.pdr.0000250206.79628.66
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.pdr.0000250206.79628.66
This article is cited by
-
Lung abnormalities do not influence aerobic capacity in school children born preterm
European Journal of Applied Physiology (2021)
-
Outcome of very low birthweight infants after introducing a new standard regime with the early use of nasal CPAP
European Journal of Pediatrics (2008)



 VLBW+DEX group,
VLBW+DEX group,  VLBW without DEX group, ▪ TERM group.
VLBW without DEX group, ▪ TERM group.


