Abstract
To characterize the thromboxane A2 (TXA2) -induced resistance to the vasodilator effects of the nitric oxide (NO)/cGMP pathway in pulmonary arteries, we have studied the effects of the NO donor sodium nitroprusside on intracellular calcium concentration ([Ca2+]i) and contractile force recorded simultaneously in isolated piglet pulmonary arteries loaded with fura-2 and contracted with norepinephrine or the TXA2 mimetic U46619 and by activation of protein kinase C (PKC) with phorbol 12-myristate 13-acetate. In the TXA2 mimetic- and phorbol 12-myristate 13-acetate plus norepinephrine-stimulated arteries, nitroprusside exhibited lower vasodilator efficacy (and lower potency in the TXA2 mimetic-stimulated arteries) but similar reductions in [Ca2+]i compared with arteries activated by norepinephrine. The nonselective serine/threonine kinase inhibitor staurosporine, but not the selective inhibitor of PKC bisindolylmaleimide, potentiated the relaxation of nitroprusside in the TXA2 mimetic-stimulated arteries. In conclusion, the resistance to NO/cGMP-induced vasodilation in arteries stimulated by TXA2 and PKC involves a reduced ability of the Ca2+-independent mechanisms for smooth muscle vasodilation. The resistance to NO in arteries stimulated by TXA2 is sensitive to staurosporine but not to bisindolylmaleimide, suggesting the involvement of an activation of a serine/threonine kinase distinct from PKC.
Similar content being viewed by others
Main
NO plays a key role in the regulation of vascular smooth muscle tone through the activation of the soluble guanylate cyclase and the subsequent increase in cGMP (1, 2). Alterations of the NO/cGMP pathway have been associated with a number of vascular diseases. In the pulmonary circulation, NO is crucial for maintaining low vascular resistances and arterial pressure (3, 4). Reduced NO or cGMP activities have been associated with the high pulmonary pressure during fetal life (5) and PPHN (6–8), as well as primary and secondary pulmonary hypertension (9, 10). In addition, inhaled NO is widely used in the treatment of pulmonary hypertension in both adults and neonates (11–13).
Increased activity of the potent pulmonary vasoconstrictor TXA2 has also been implicated in several forms of human and experimental pulmonary hypertension including those induced by sepsis (14), heparin/protamine (15), leukotriene D4 (16), microembolism (17), and ischemia-reperfusion (18). Elevated levels of thromboxane B2, the metabolite of TXA2, have been found in patients with PPHN (19). Moreover, TXA2 may play a key role in pulmonary hypertension not only because of its potent vasoconstrictor activity but also because TXA2-induced vasoconstriction is highly resistant to the vasodilator effects of NO (20–24). Therefore, resistance of TXA2-induced pulmonary vasoconstriction to NO might be implicated in the therapeutic failure of inhaled NO in some patients with PPHN (11, 12).
The reduced vasodilator activity of NO in TXA2-induced pulmonary vasoconstriction was not related to a reduced cGMP synthesis, the initial step in the signal transduction of NO, but rather to changes downstream in the signaling cascade of cGMP (24), which involve both cytosolic [Ca2+]i-dependent and [Ca2+]i-independent pathways (25–35). At present, it is unknown whether the resistance to the NO/cGMP pathway in TXA2-stimulated arteries is dependent on alterations of [Ca2+]i or is caused by [Ca2+]i-independent effects. Inasmuch as the nonspecific protein kinase inhibitor staurosporine blunted the resistance to NO in TXA2-stimulated arteries and the protein kinase C activator PMA mimicked the TXA2-induced resistance to NO-induced vasodilation, it was suggested that protein kinase C was involved in this process (24).
Therefore, the aims of the present study were 1) to analyze whether the resistance to the vasodilator effect of the NO/cGMP pathway in TXA2-induced pulmonary vasoconstriction is associated with alterations in [Ca2+]i and 2) to further characterize the possible involvement of protein kinase C. For these purposes, we have studied the effects of the NO donor sodium nitroprusside on [Ca2+]i and contractile force recorded simultaneously in isolated piglet pulmonary arteries stimulated with the TXA2-mimetic U46619 and with the activator of protein kinase C, PMA. Norepinephrine-induced vasoconstriction was used as control, and staurosporine and bisindolylmaleimide, as nonspecific and specific inhibitors of protein kinase C, respectively.
METHODS
Tissue preparation.
Twenty-four male piglets (10–17 d, 3–5 kg) were used in this study. The procedures were approved by the Complutense University Animal Care and Use Committee. Piglets were killed in the local abattoir, and the lungs were rapidly immersed in cold (4°C) Krebs solution (composition in mM: NaCl 118, KCl 4.75, NaHCO3 25, MgSO4 1.2, CaCl2 2.0, KH2PO4 1.2, and glucose 11) and transported to the laboratory. The intrapulmonary arteries (third branch, internal diameter 1–2 mm) were carefully dissected free of surrounding tissue and cut into rings of 2–3 mm length (20, 21). The endothelium was removed by gently rubbing the intimal surface of the rings with a metal rod. The procedure of endothelium removal was verified by the absence of a vasodilator response to acetylcholine (10−5 M) in arteries stimulated by norepinephrine.
Simultaneous measurements of [Ca2+]i and tension.
Endothelium-denuded rings were inverted and incubated for 4–6 h at room temperature in Krebs solution containing fura-2 acetoxymethylester (5 × 10−6 M) and Cremophor EL (0.05%). Thereafter, rings were mounted between two hooks under 0.5 g of tension in a 5-mL organ bath filled with Krebs solution at 37°C and gassed with a 95% O2/5%CO2 gas mixture in the absence of fura-2. The bath was part of a fluorimeter (CAF 110, Jasco, Tokyo, Japan), which allows us to estimate changes in the fluorescence intensity of fura-2 simultaneously with contractile force by an isometric force transducer as previously described (36, 37). The luminal face of the rings was alternately illuminated (128 Hz) with two excitation wavelengths (340 and 380 nm) from a xenon lamp coupled with two monochromators. The emitted fluorescent light at the two excitation wavelengths (F340 and F380) was measured by a photomultiplier through a 500-nm filter and recorded together with the force data by using data acquisition hardware (Mac Lab, model 8, AD Instruments Pty Ltd., Castle Hill, Australia) and data recording software (Chart v3.2, AD Instruments Pty Ltd.). The absolute values of [Ca2+]i were estimated from the ratio of emitted fluorescence obtained at the two excitation wavelengths (F340/F380) using the Grynkiewicz equation as reported by Kanaide (38). The maximal and minimal F340 and F380 values for this equation were obtained by treatment with ionomycin (1.4 × 10−6 M) and then with EGTA (8 mM) as previously described (38).
After equilibration for 30–45 min, vessels were initially stimulated with 80 mM KCl for 10–15 min, which induced a sustained increase in [Ca2+]i and force. After washing in normal Krebs solution, the rings were stimulated with norepinephrine (10−5 M) or the TXA2 mimetic U46619 (10−7 M), and a concentration-response curve to nitroprusside (10−8 M to 3 × 10−5 M) was constructed by cumulative addition of the drug. The concentrations of norepinephrine and U46619 were chosen because in previous studies they produced a similar increase in contractile force (approximately 60–80% of the maximal response to U46619) (20, 24). In some experiments, PMA (10−7 M) was added 25 min before stimulation with norepinephrine, and in another set of experiments, staurosporine (10−7 M) or bisindolylmaleimide (10−6 M) was added 25 min before stimulation with U46619. Because staurosporine decreased the contractile response to U46619, in these experiments the concentration of U46619 was raised to 2 × 10−7 M to reach a contractile force similar to that induced by U46619 in the absence of the drug.
Drugs.
The following drugs were used: (−)-norepinephrine bitartrate, sodium nitroprusside, PMA, thapsigargin, staurosporine, and bisindolylmaleimide I (Sigma Chemical Co., Alcobendas, Madrid, Spain), U46619 (Alexis Corporation, Läufelfingen, Switzerland) and fura-2 acetoxymethylester (1 mM solution in DMSO, Calbiochem, La Jolla, CA, U.S.A.). Drugs were dissolved initially in distilled deionized water (except for staurosporine, thapsigargin, bisindolylmaleimide, and PMA, which were dissolved in DMSO) to prepare a 10−2 M or 10−3 M stock solution, and further dilutions were made in Krebs solution. The concentrations are expressed as final molar concentration in the tissue chamber.
Statistical analysis.
Results are expressed as mean ± SEM where n equals the number of animals. The results of both [Ca2+]i and force are expressed as a percentage, considering the values at rest in normal Krebs solution and after 80 mM KCl-induced stimulation to be 0% and 100%, respectively (28). Individual cumulative concentration-response curves were fitted to a logistic equation. The drug concentration exhibiting 50% of the Emax was calculated from the fitted concentration-response curves for each ring and expressed as pD2. Statistically significant differences among groups were calculated by one-way ANOVA followed by a Newman-Keuls't test, with p < 0.05 considered statistically significant.
RESULTS
Responses to norepinephrine, the TXA2 mimetic U46619, and PMA.
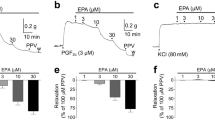
In fura-2 unloaded arteries, 80 mM KCl induced a contractile response and weakly increased both the F340 and F380 signals, but no change was observed in the ratio F340/F380. Stimulation of fura-2-loaded pulmonary arteries with 80 mM KCl induced a sustained increase in the F340/F380 signal of 16.7 ± 1.9% above resting values. Absolute values of [Ca2+]i under basal conditions and after stimulation with KCl were 105 ± 19 and 196 ± 36 nM, respectively, and the sustained contractile response averaged 418 ± 37 mg in 38 arteries (not significantly different from that in fura-2 unloaded arteries). As shown in Figure 1, both norepinephrine (10−5 M) and the TXA2 mimetic U46619 (10−7 M) induced a fast increase in [Ca2+]i followed by a decay to a lower sustained level (Fig. 1, A and B). In contrast, PMA (10−7 M) had no effect on [Ca2+]i (−3.7 ± 1.1% of the response to KCl, p > 0.05) but induced a slowly developing contractile response (16 ± 3% of the response to KCl at 30 min, p < 0.05, Fig. 1C). In the presence of phorbol esters, the increase in [Ca2+]i induced by norepinephrine was strongly inhibited, which is consistent with data previously described in sheep cerebral arteries, possibly because of a reduction in phospholipase C activity (39). Nevertheless, application of thapsigargin at the end of the experiment increased [Ca2+]i to values similar to those induced by KCl (Fig. 1C). Figure 2 shows that the Ca2+–force relationship constructed with increasing concentrations of U46619 (10−9 M to 10−7 M) was very similar to that for norepinephrine (10−8 M to 10−5 M). At 10−7 M U46619 and 10−5 M norepinephrine, the force relative to [Ca2+]i (% force/% [Ca2+]i) was 2.1 and 2 times greater, respectively, than that induced by 80 mM KCl (p < 0.05). In the presence of PMA, the norepinephrine-induced increase in [Ca2+]i was strongly reduced, but the contractile force was not significantly different from that in the absence of PMA and thus a 4.5-fold increase in the force relative to [Ca2+]i compared with PMA-untreated arteries was observed.
Representative experiments showing the effects of norepinephrine (A), U46619 (B), and PMA plus norepinephrine (C) and the inhibitory effects of nitroprusside on [Ca2+]i (F340/F380, upper traces) and contractile force (lower traces) in endothelium-denuded pulmonary arteries. Arteries were initially stimulated by 80 mM KCl, then with norepinephrine (10−5 M), U46619 (10−7 M), or PMA (10−7 M) plus norepinephrine (10−5 M), as indicated by the bars, and thereafter a cumulative concentration-response curve to nitroprusside (10−8 M, 10−7 M, 10−6 M, 10−5 M, and 3 × 10−5 M) was constructed by cumulative addition of the drug as indicated by the arrows. C shows the effects of thapsigargin (THAP, 10−6 M) when added at the end of the experiment. The vertical calibration bars for force recordings indicate 500 mg. The traces of F340/F380 represent arbitrary units.
[Ca2+]i-force relationships constructed by increasing concentrations of norepinephrine (10−8 M to 10−5 M) and U46619 (10−9 M to 10−7 M) or by a single concentration of PMA (10−7 M) plus norepinephrine (10−5 M). The logarithm of the concentrations of norepinephrine or U46619 are indicated at each point. The results (mean ± SEM, 4–7 experiments) are expressed as a percentage of the response to 80 mM KCl.
Effects of nitroprusside.
Cumulative addition of nitroprusside induced a concentration-dependent reduction in [Ca2+]i and relaxation (Figs. 1 and 3, Table 1). Nitroprusside reverted the increase in [Ca2+]i induced by norepinephrine, the TXA2 mimetic, and PMA plus norepinephrine with a similar potency and efficacy (i.e. similar pD2 and Emax values), even when marked differences in the preexisting [Ca2+]i level were observed in PMA plus norepinephrine-treated arteries (Fig. 3A). Nitroprusside relaxed norepinephrine-stimulated arteries in parallel to the reduction in [Ca2+]i (Fig. 3, A and B). In contrast, nitroprusside produced only a partial relaxant effect on U46619- and PMA plus norepinephrine-induced contractions (p < 0.05 versus norepinephrine and p < 0.05 versus [Ca2+]i). The reduction in Emax was accompanied by a significant reduction in the pD2 value in U46619-treated but not in PMA plus norepinephrine-treated arteries (Table 1). Figure 3C shows the plot of the relaxation as a function of [Ca2+]i reduction induced by nitroprusside, i.e. the [Ca2+]i-force relationship. It can be observed that in norepinephrine-stimulated arteries, the [Ca2+]i-force relationship was almost linear. In contrast, in arteries stimulated by U46619, low concentrations of nitroprusside (up to 10−7 M) induced smaller relaxant effects for a given reduction in [Ca2+]i compared with norepinephrine-stimulated arteries, whereas at higher concentrations of nitroprusside the reduction in [Ca2+]i was accompanied by significant relaxation. At 3 × 10−5 M nitroprusside, [Ca2+]i values were similar to resting levels, but there was still a component (22 ± 3%) of contraction remaining. Therefore, the [Ca2+]i-force relationship showed a nonlinear shape, i.e. there was a dissociation between [Ca2+]i and contractile force. In PMA plus norepinephrine-treated arteries, the [Ca2+]i values before the addition of nitroprusside were only 9 ± 3% of the response to 80 mM KCl, and after 3 × 10−5 M nitroprusside, they were reduced to −7 ± 3% (not significantly different from baseline), i.e. the relaxant effect of nitroprusside was accompanied of absolute changes in [Ca2+]i much smaller than those observed in norepinephrine- and U46619-stimulated arteries. The [Ca2+]i-force relationship was almost linear, but it was significantly steeper (p < 0.05) compared with arteries treated with norepinephrine alone.
Effects of nitroprusside (10−8 M to 3 × 10−5 M) on [Ca2+]i (A) and force (B) in endothelium-denuded pulmonary arteries stimulated by norepinephrine (10−5 M), U46619 (10−7 M), and PMA (10−7 M) plus norepinephrine (10−5 M). The experiments were performed as shown in Figure 1. The insets in A and B show the same results expressed as a percentage of control values before the addition of nitroprusside. C, [Ca2+]i- force relationship obtained from data in A and B. Control values before the addition of nitroprusside are indicated by C, and the logarithm of the concentration of nitroprusside is at each point. The symbols represent the mean ± SEM of 6–9 experiments. *p < 0.05 U46619 vs norepinephrine, #p < 0.05 PMA plus norepinephrine vs norepinephrine.
Effects of staurosporine and bisindolylmaleimide.
The nonselective protein kinase inhibitor staurosporine (10−7 M) or the selective protein kinase C inhibitor bisindolylmaleimide (10−6 M) had no measurable effect on baseline [Ca2+]i or force. In the experiments with staurosporine the concentration of U46619 was raised to 2 × 10−7 M to reach an equivalent contractile response to that of 10−7 M U46619 in the absence of the drug. The increases in [Ca2+]i and contractile force induced by U46619 in the presence of the drugs were not significantly different from those induced in their absence, and, therefore, the force relative to [Ca2+]i was not significantly different (Fig. 4). Cumulative addition of nitroprusside induced a concentration-dependent decrease in [Ca2+]i, which was similar in control and in staurosporine- or bisindolylmaleimide-treated arteries (Fig. 4B). However, there was a significant increase in the maximal relaxant response to nitroprusside in staurosporine- but not in bisindolylmaleimide-treated arteries (Fig. 4C, Table 1).
Effects of staurosporine (10−7 M) and bisindolylmaleimide (10−6 M) on the U46619-induced increase in [Ca2+]i (A;left), force (A;middle), and the ratio of both variables (A;right) and on nitroprusside-induced [Ca2+]i-lowering (B) and relaxation (C) in endothelium-denuded pulmonary arteries stimulated by U46619. The insets in B and C show the same results expressed as a percentage of control values before the addition of nitroprusside. The results (mean ± SEM of 5–6 experiments) are expressed as a percentage of the response to 80 mM KCl. *p < 0.05 vs control.
DISCUSSION
In the present study we have analyzed the effects of the NO donor nitroprusside on [Ca2+]i and contractile force in endothelium-denuded piglet pulmonary arteries activated by the TXA2-mimetic U46619 or by the protein kinase C activator PMA. Norepinephrine was used as a control vasoconstrictor. The results can be summarized as follows. In arteries stimulated by the TXA2 mimetic or PMA plus norepinephrine, nitroprusside induced only a partial relaxation despite a similar potency and efficacy in lowering [Ca2+]i compared with norepinephrine-stimulated arteries. The nonselective protein kinase inhibitor staurosporine but not the selective protein kinase C inhibitor bisindolylmaleimide increased the maximal relaxant response to nitroprusside in the TXA2 mimetic-stimulated arteries, although neither of them modified the nitroprusside-induced [Ca2+]i-lowering effects.
The regulation of vascular smooth muscle tone depends primarily on changes in [Ca2+]i, which controls myosin light chain kinase activity 25. However, in recent years, Ca2+-independent mechanisms (i.e. Ca2+ sensitization) involving changes in myosin light chain phosphatase activity or actin-linked regulatory mechanisms have also been reported to play an important role (25, 40). The Ca2+ sensitization induced by phorbol esters (which directly stimulate protein kinase C) and receptor agonists such as TXA2 and norepinephrine have been reported elsewhere (25, 40, 41). Likewise, in the present study, both the TXA2 mimetic and norepinephrine induced a substantial greater contraction relative to the increase in [Ca2+]i than 80 mM KCl. The force relative to [Ca2+]i was similar for the TXA2 mimetic and norepinephrine (about twofold increase over KCl), i.e. they produced similar Ca2+ sensitization. Furthermore, the Ca2+-force relationships were similar for the whole concentration-response curves to both agonists. However, in the presence of PMA, norepinephrine induced a weak increase in [Ca2+]i, but the contractile response was not significantly different from that observed in the absence of PMA. Thus, force relative to [Ca2+]i (Ca2+ sensitization) in PMA plus norepinephrine-stimulated arteries was much higher than in the TXA2 mimetic- or norepinephrine-stimulated arteries.
Nitroprusside causes vascular smooth muscle relaxation by releasing NO, which, in turn, activates guanylate cyclase and increases intracellular cGMP levels (26). cGMP and cGMP-activated protein kinase may control a large number of cellular activities to regulate vascular smooth muscle tone (2, 28). The relative role of these mechanisms of action varies widely depending on the vascular tissue. In piglet pulmonary arteries, nitroprusside-induced relaxation is blunted by inhibition of guanylate cyclase and has been attributed to both [Ca2+]i-dependent and -independent mechanisms (24, 30).
Porcine intrapulmonary arteries stimulated by the TXA2 mimetic showed smaller relaxant responses to nitroprusside and other agents stimulating the NO/cGMP pathway than arteries stimulated by norepinephrine (24). This effect was specific for piglet pulmonary arteries, inasmuch as it was not seen in piglet mesenteric and coronary arteries and it was specific for the cGMP pathway because similar relaxant responses were observed for the activator of the cAMP pathway, forskolin, in the TXA2 mimetic- and norepinephrine-stimulated arteries (24). In the present study, we have demonstrated that the TXA2 mimetic- and PMA plus norepinephrine-induced vasoconstrictions are relatively resistant to vasodilate in response to nitroprusside, although the ability of this NO donor to lower [Ca2+]i is similar regardless of the vasoconstrictor agonist. Therefore, the difference in the nature of the stimulation only influences the [Ca2+]i-independent component of NO-induced vasodilation. In the case of PMA plus norepinephrine, this difference in the stimulation process could be attributed to the different degree of Ca2+ sensitization produced by PMA plus norepinephrine (i.e. the contractile responses occurred with very weak changes in [Ca2+]i) compared with norepinephrine alone. In contrast, norepinephrine and TXA2 produced similar Ca2+ sensitization. Therefore, we attempted to further characterize the signaling pathway of TXA2 involved in the reduced response to NO by using the protein kinase inhibitors staurosporine and bisindolylmaleimide. The nonselective serine/threonine protein kinase inhibitor staurosporine induced no change in the [Ca2+]i-lowering effect of nitroprusside but increased nitroprusside-induced relaxation, suggesting the involvement of a protein kinase. However, several findings suggest that this serine/threonine protein kinase is not protein kinase C:1) the TXA2 mimetic did not produce as high a sensitization as the protein kinase C stimulator PMA (Fig. 2);2) the potency (pD2) of nitroprusside to relax pulmonary arteries was reduced in the TXA2 mimetic- but not in PMA plus norepinephrine-induced contractions (Table 1);3) a clearly different [Ca2+]i-force relationship for the effects of nitroprusside was observed in arteries stimulated by the TXA2 mimetic and PMA plus norepinephrine (Fig. 3C); and 4) the selective protein kinase C inhibitor bisindolylmaleimide did not modify the relaxant effects of nitroprusside in the TXA2 mimetic-stimulated arteries (Fig. 4). Thus, the signaling of TXA2, involving a staurosporine-sensitive but bisindolylmaleimide-insensitive mechanism, reduces the sensitivity of the [Ca2+]i-independent relaxant effects of NO.
In conclusion, the pulmonary vasoconstriction induced by the TXA2 mimetic U46619 or by activation of protein kinase C is partially resistant to the relaxant responses induced by the NO donor nitroprusside. However, nitroprusside fully reverted the increase in [Ca2+]i induced by the vasoconstrictor stimuli. Therefore, the resistance to NO/cGMP-induced vasodilation involves Ca2+-independent mechanisms. The different pattern of the NO/cGMP resistance induced by the TXA2-mimetic compared with that induced by the protein kinase C activator PMA, together with the lack of effect of the selective protein kinase C inhibitor bisindolylmaleimide, indicates that protein kinase C is not involved and suggests a role for other serine/threonine kinase(s).
Abbreviations
- [Ca2+]i:
-
intracellular calcium concentration
- Emax:
-
maximal effect
- F340/F380:
-
ratio of fluorescence at 340 and 380 nm (index of cytosolic Ca2+)
- NO:
-
nitric oxide
- pD2:
-
negative log molar of the drug concentration exhibiting 50% of the Emax
- PMA:
-
phorbol 12-myristate 13-acetate
- PPHN:
-
persistent pulmonary hypertension of the newborn
- TXA2:
-
thromboxane A2
- U46619:
-
9,11-dideoxy-11α,9α-epoxymethano-prostaglandin F2α (thromboxane mimetic)
References
Moncada S, Palmer RMJ, Higgs EA . Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 1991 43: 109–142
Warner TD, Mitchell JA, Sheng H, Murad F Effects of cyclic GMP on smooth muscle relaxation. Adv Pharmacol Hige 26: 171–194
Cremona G, Dinh-Xuan AT, Higenbottam TW . Endothelium-derived relaxing factor and the pulmonary circulation. Lung 1991 169: 185–202
Barnes PJ, Liu SF . Regulation of pulmonary vascular tone. Pharmacol Rev 1995 47: 87–131
Abman SH, Chatfield B, Hall SL, McMurtry IF . Role of endothelium-derived releasing factor during the transition of the pulmonary circulation at birth. Am J Physiol 1990 259: H1921–H1927
McQueston JA, Kinsella JP, Ivy DD, McMurtry IF, Abman SH . Chronic pulmonary hypertension in utero impairs endothelium-dependent vasodilation. Am J Physiol 1995 268: H288–H294
Villamor E, Le Cras T, Horan M, Halbower AC, Tuder RM, Abman SH . Chronic intrauterine pulmonary hypertension impairs endothelial nitric oxide synthase in the ovine fetus. Am J Physiol 1997 16: L1013–L1020
Villanueva MET, Zaher FM, Svinarich DM, Konduri GG . Decreased gene expression of the endothelial nitric oxide synthase in newborns with persistent pulmonary hypertension. Pediatr Res 1998 44: 338–343
Giaid A, Saleh D . Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med 1995 333: 214–221
Dinh-Xuan AT, Higenbottam TW, Clelland C, Pepke-Zaba J, Cremona G, Butt AY, Large SR, Wells FC, Wallwork J . Impairment of endothelium-dependent pulmonary artery relaxation in chronic obstructive lung disease. N Engl J Med 1991 324: 1539–1547
Roberts JD, Fineman JR, Morin FC, Shaul PW, Rimar S, Schreiber MD, Polin RA, Zwass MS, Zayec MM, Grossi Heymann MA, Zapol WM . Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. N Engl J Med 1997 336: 605–610
Neonatal Inhaled Nitric Oxide Study Group 1997 Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. N Engl J Med 336: 597–604
Abman SH, Kinsella JP . Inhaled nitric oxide for persistent pulmonary hypertension of the newborn: the physiology matters. Pediatrics 1995 96: 1153–1155
Weitzberg E, Lundberg JM, Rudehill A . Inhibitory effects of diclofenac on the endotoxin shock response in relation to endothelin turnover in the pig. Acta Anaesthesiol Scand 1995 39: 50–59
Montalescot G, Lowenstein E, Ogletree ML, Greene EM, Robinson DR, Hartl K, Zapol WM . Thromboxane receptor blockade prevents pulmonary hypertension induced by heparin-protamine reactions in awake sheep. Circulation 1990 82: 1765–1777
Noonan TC, Malik AB . Pulmonary vascular response to leukotriene D4 in unanesthetized sheep: role of thromboxane. J Appl Physiol 1986 60: 765–769
Garcia-Szabo RR, Johnson A, Malik AB . Thromboxane increases pulmonary vascular resistance and transvascular fluid and protein exchange after pulmonary microembolism. Prostaglandins 1988 35: 707–721
Zamora CA, Baron DA, Heffner JE . Thromboxane contributes to pulmonary hypertension in ischemia-reperfusion lung injury. J Appl Physiol 1993 74: 224–229
Dobyns EL, Wescott JY, Kennaugh JM, Ros MN, Stenmark KR . Eicosanoids decrease with successful extracorporeal membrane oxygenation therapy in neonatal pulmonary hypertension. Am J Respir Crit Care Med 1994 149: 873–880
Pérez-Vizcaíno F, Villamor E, Fernandez del Pozo B, Moro M, Tamargo NJ . Lack of endotoxin-induced hyporesponsiveness to U46619 in isolated neonatal porcine pulmonary but not mesenteric arteries. J Vasc Res 1996 33: 249–257
Villamor E, Pérez-Vizcaíno F, Moro M, Tamargo J . Effects of group B streptococcus on the responses to U46619, endothelin and noradrenaline in isolated intrapulmonary and mesenteric arteries of piglets. Pediatr Res 1996 40: 827–833
Griffiths MJ, Curzen NP, Mitchell JA, Evans TW . In vivo treatment with endotoxin increases rat pulmonary vascular contractility despite iNOS induction. Am J Respir Crit Care Med 1997 156: 654–658
Resta TC, O'Donaughy TL, Earley S, Chicoine LG, Walker BR . Unaltered vasoconstrictor responsiveness after iNOS inhibition in lungs from chronically hypoxic rats. Am J Physiol 1999 276: L122–L130
Pérez-Vizcaíno F, Villamor E, Duarte J, Tamargo J . Involvement of protein kinase C in reduced relaxant responses to the NO/cyclic GMP pathway in piglet pulmonary arteries contracted by the thromboxane A2 mimetic U46619. Br J Pharmacol 1997 121: 1323–1333
Karaki H, Ozaki H, Hori M, Mitsui-Saito M, Amano K-I, Harada K-I, Miyamoto S, Nakazawa H, Won K-J, Sato K . Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev 1997 49: 157–230
Rapoport RM, Schwartz K, Murad F . Effect of sodium-potassium pump inhibitors and membrane-depolarizing agents on sodium nitroprusside-induced relaxation and cyclic guanosine monophosphate accumulation in rat aorta. Circ Res 1985 57: 164–170
Kitazawa T, Lee M, Zhang M, Masuo M, Li L . cGMP-induced Ca2+ desensitization of contraction and myosin light chain phosphorylation in permeabilized smooth muscle. Biophys J 1996 70: A383( abstr)
Lincoln TM, Komalavilas P, Cornwell TL . Pleiotropic regulation of vascular smooth muscle tone by cyclic GMP-dependent protein kinase. Hypertension 1994 23: 1141–1147
Ghisdal P, Gomez J-P, Morel N . Action of a NO donor on the excitation-contraction pathway activated by noradrenaline in rat superior mesenteric artery. J Physiol (Lond) 2000 522: 83–96
Cogolludo AL, Pérez-Vizcaíno F, Zaragoza-Arnaez F, Ibarra M, López-López JG, López-Miranda V, Tamargo J . Mechanisms involved in SNP-induced relaxation and [Ca2+]i reduction in piglet pulmonary and systemic arteries. Br J Pharmacol 2001 132: 959–967
Cohen R, Weisbrod R, Gericke M, Yaghoubi M, Bierl C, Bolotina V . Mechanism of nitric oxide vasodilation: refilling of intracellular stores by sarcoplasmic reticulum ATPase and inhibition of stores-operated Ca2+ influx. Circ Res 1999 84: 210–219
Archer SL, Huang JMC, Hampl V, Nelson DP, Shultz PJ, Weir EK . Nitric oxide and cyclic GMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cyclic GMP-dependent protein kinase. Proc Natl Acad Sci USA 1994 91: 7583–7587
Conti MA, Adelstein RS . The relationship between calmodulin binding and phosphorylation of smooth muscle myosin kinase by the catalytic subunit of 3′:5′ cAMP-dependent protein kinase. J Biol Chem 1981 256: 3178–3181
Wu X, Somlyo AV, Somlyo AP . Cyclic GMP-dependent stimulation reverses G-protein-coupled inhibition of smooth muscle myosin light chain phosphatase. Biochem Biophys Res Commun 1996 220: 658–663
Woodrum DA, Brophy CM, Wingard CJ, Beall A, Rasmusen H . Phosphorylation events associated with cyclic nucleotide-dependent inhibition of smooth muscle contraction. Am J Physiol 1999 277: H931–H933
Pérez-Vizcaíno F, Fernández C, Fernández del Pozo B, Cogolludo A Zaragozá F, Tamargo J . Vasoconstrictor and vasodilator effects of disopyramide in isolated rat vascular smooth muscle. J Cardiovasc Pharmacol 1998 32: 754–752
Pérez-Vizcaíno F, Cogolludo A, Tamargo J . Modulation of arterial Na+/K+-ATPase-induced [Ca2+]i reduction and relaxation by norepinephrine, ET-1 and PMA. Am J Physiol 1999 276: H651–H657
Kanaide H . Measurement of [Ca2+]i in smooth muscle strips using front surface fluorimetry. Methods Mol Biol 1999 114: 269–277
Longo LD, Zhao Y, Long W, Miguel C, Windemuth RS, Cantwell A-M, Nanyonga AT, Saito T, Zhang L . Dual role of PKC in modulating pharmacomechanical coupling in fetal and adult cerebral arteries. Am J Physiol 2000 279: R1419–R1429
Somlyo AP, Somlyo AV . Signal transduction by G-proteins, Rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol (Lond) 2000 522: 177–185
Himpens B, Kitazawa T, Somlyo A . Agonist-dependent modulation of Ca2+ sensitivity in rabbit pulmonary artery smooth muscle. Pflugers Arch 1990 417: 21–28
Acknowledgements
The authors thank C. Rivas for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by a CICYT (SAF 99/0069) grant. F.P.-V. and A.C. are supported by grants from the Comunidad Autónoma de Madrid.
Rights and permissions
About this article
Cite this article
Pérez-Vizcaíno, F., Cogolludo, A., Ibarra, M. et al. Pulmonary Artery Vasoconstriction but not [Ca2+]i Signal Stimulated by Thromboxane A2 Is Partially Resistant to NO. Pediatr Res 50, 508–514 (2001). https://doi.org/10.1203/00006450-200110000-00014
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200110000-00014