Abstract
Free iron chelation after hypoxia-ischemia can reduce free radical-induced damage to brain cell membranes and preserve electrical brain activity. We investigated whether chelation of free iron with deferoxamine (DFO) preserved cortical cell membrane activity of Na+,K+-ATPase and electrocortical brain activity (ECBA) of newborn lambs during early reperfusion after severe hypoxia-ischemia. Hypoxia was induced in 16 lambs by decreasing the fraction of inspired oxygen to 0.07 for 30 min, followed by a 5-min period of hypotension (mean arterial blood pressure <35 mm Hg). ECBA (in microvolts) was measured using a cerebral function monitor. Immediately after hypoxia and additional ischemia, eight lambs received DFO (2.5 mg/kg, i.v.), and seven lambs received a placebo (PLAC). Two lambs underwent sham operation. One hundred eighty minutes after completion of hypoxia and ischemia, the brains were obtained and frozen. Na+,K+-ATPase activity was measured in the P2 fraction of cortical tissue. Na+,K+-ATPase activity was 35.1 ± 7.4, 42.0 ± 7.6, and 40.7 ± 1.4 μmol inorganic phosphate/mg protein per hour in PLAC-treated, DFO-treated, and sham-operated lambs, respectively (p < 0.05: DFO versus PLAC). ECBA was 11.2 ± 6.1, 14.8 ± 4.8, and 17.5±.0.5 μV in PLAC-treated, DFO-treated, and sham-operated lambs, respectively (p = 0.06: DFO versus PLAC). Na+,K+-ATPase activity correlated with ECBA at 180 min of reperfusion (r = 0.85, p < 0.001). We conclude that Na+,K+-ATPase activity of cortical brain tissue was higher in DFO-treated lambs compared with PLAC-treated animals during the early reperfusion phase after severe hypoxia-ischemia, suggesting a reduction of free radical formation by DFO. Furthermore, a positive relationship was found between Na+,K+-ATPase activity and ECBA.
Similar content being viewed by others
Main
Hypoxia and ischemia during perinatal asphyxia give rise to an inadequate substrate supply to brain tissue, resulting in damage of neuronal cells. Although recovery of oxygenation and perfusion of the brain is mandatory to prevent further damage, reoxygenation of previously ischemic brain tissue has increasingly been recognized as an important mechanism for additional injury to the neuronal cells and the cerebral microcirculation (1, 2). Production of reactive oxygen species in the early reperfusion phase plays a substantial role in this type of brain cell damage: Reactive oxygen species such as superoxide and hydrogen peroxide can be converted into the highly reactive hydroxyl radical by transition metals, in particular free iron, ultimately leading to lipid peroxidation of the brain cell membrane and cellular damage (3). In recent experimental and clinical studies, we showed that chelation of free iron prevented posthypoxic-ischemic hypoperfusion and metabolic derangements of the brain and preserved ECBA (4, 5). We also found that the free iron chelator DFO effectively lowered free iron in cortical brain tissue (6).
Inasmuch as the transmembrane enzyme Na+,K+-ATPase is very susceptible to free radical-related lipid peroxidation (7, 8), we investigated in the present study whether DFO prevented free radical-induced alterations of the brain cell membrane after global hypoxia and ischemia, simulating severe birth asphyxia, by measuring cortical cell membrane Na+,K+-ATPase activity in the early reoxygenation and reperfusion phase in newborn lambs. As Na+,K+-ATPase is important in maintaining membrane potentials, a second objective of this study was to investigate the relation between cortical cell membrane Na+,K+-ATPase activity and ECBA in the early reperfusion phase using cerebral function monitoring.
METHODS
Animal preparation.
Eighteen newborn lambs aged 7.5 ± 1 d (mean ± SD) and weighing 4.1 ± 1.3 kg were used. The lambs were from an inbred strain. Surgical and experimental procedures were approved by the Animal Research Committee of the Leiden University Hospital. The lambs were anesthetized with a bolus of ketamine hydrochloride (3 mg/kg, i.v.) and xylazine (1 mg/kg, i.v.) and paralyzed with pancuronium bromide (0.2 mg/kg, i.v.). The lambs were ventilated with oxygen and air, using a continuous-flow, pressure-controlled ventilator (Bourns BP 200, Bear Medical Systems Inc., Riverside, CA, U.S.A.). Ventilation was adjusted to maintain arterial Po2 and Pco2 in the normal range. The right femoral artery was used for determining aortic blood pressure and for the sampling of arterial blood gases and pH. Both femoral veins were used for withdrawal of blood and infusion of drugs. An i.v. infusion of 5% glucose in 0.9% NaCl was continued throughout the study at 15 mL/kg per hour. Arterial blood gases and pH were measured using a Corning 178 pH/blood gas analyzer (Corning, Halstead, U.K.). Appropriately sized ultrasonic flow transducers (Transonic Systems Inc., Ithaca, NY, U.S.A.) were applied to fit around the carotid arteries for measurement of the V˙car by the transit-time technique (9). Changes in brain blood flow were assessed by changes in V˙car (in milliliters per minute). An earlier study showed a close linear relationship and acceptable assessment between V˙car and actual brain blood flow as determined by radioactive microspheres (10). After completion of the surgical procedure, the lambs had a 3-h baseline period to achieve hemodynamic stability and to wash out ketamine. Throughout the experiment, the lambs were kept sedated with xylazine, and the incision wounds were sprayed with 1% lidocaine at regular intervals.
Continuous measurement of ECBA.
Changes in ECBA were monitored using a filtered and selectively amplified one-channel cerebral function monitor (Lectromed, Oxford Instruments, Oxford, U.K.), described by Prior and Maynard (11). The cerebral function monitor has a special filter, which sharply attenuates frequencies <2 and >15 Hz, giving an amplitude-integrated recording that contains the main EEG frequencies, but with little disturbance from artifacts. The EEG signal was obtained from a pair of silver-chloride disk electrodes, placed with electrode cream at the P3 and P4 position of the 10–20 International System, i.e. in the left and right parietal region (11, 12). The ECBA was recorded on a semilogarithmic scale (0–100 μV). The paper speed was 2 mm/min. Simultaneously with the amplitude curve, an impedance curve records the reliability of the signal by a reference electrode positioned anterior to the scalp and shows artifacts from movement, experimental procedures, or loose electrodes. The mean voltage of the ECBA determined at 180 min of reperfusion for a period of 2 min, aortic blood pressure, V˙car, and the cerebral function monitor signal were measured continuously, digitized with a sample frequency of 200 Hz, and stored on a personal computer.
Experimental procedure.
Severe hypoxia was induced in 16 of 18 lambs by ventilation with 6 to 8% oxygen supplemented with a mixture of 10% CO2 in N2 during 30 min, followed by a 5-min period of hypotension (mean aortic blood pressure <35 mm Hg), which was achieved by careful withdrawal of blood (50 to 150 mL). We followed this procedure to emulate the clinical situation during severe perinatal asphyxia. On resuscitation, after completion of the hypoxia and additional ischemia, eight lambs received an i.v. infusion with a placebo (PLAC) of 30 mL of 0.9% NaCl, and eight lambs received 2.5 mg/kg DFO (deferoxamine-mesylate) i.v. in 30 mL 0.9% NaCl. Resuscitation was performed in a way similar to the resuscitation protocol in the neonatal unit. The blood withdrawn to achieve hypotension was reinfused immediately after the completion of the hypoxia and additional ischemia. Sodium bicarbonate was supplemented to correct low arterial pH caused by metabolic acidosis. Two lambs served as sham-operated animals (SHAM), underwent the animal preparation as described above, and underwent the same treatment as the study groups except for the hypoxia and additional ischemia.
After 3 h posthypoxia-ischemia, cortical brain tissue was collected and stored until analysis (see below). Cortical brain tissue was frozen in liquid nitrogen and stored immediately at −70°C.
Measurement of cortical brain cell membraneNa+,K+-ATPase activity.
Na+,K+-ATPase activity was measured in the P2 membrane fraction. Membranes were prepared as described previously (7). Vesicle formation during this procedure is very limited (13). The Na+,K+-ATPase activity was determined by the rate of ATP hydrolysis in a 1.0-mL reaction mixture containing NaCl 100 mM, KCl 20 mM, MgCl2 3 mM, Tris-ATP 3 mM, Tris HCl buffer (pH 7.4) 50 mM, and membrane protein 100 μg. The reaction was carried out at 37°C in the presence and absence of 1.0 mM ouabain for 5 min, during which period ATP hydrolysis was linear. The reaction was stopped by addition of 0.5 mL of ice-cold 12.5% trichloroacetic acid. The samples were kept on ice and centrifuged at 2000 ×g for 15 min, and the supernatant was analyzed for liberated Pi. The ouabain-sensitive activity was referred to as Na+,K+-ATPase activity and expressed as micromoles of Pi per milligram protein per hour (14). The protein content of the tissue was assessed by the method of Lowry et al. (15).
Statistical analysis.
Unpaired t tests or Mann-Whitney U tests and ANOVA for repeated measurements, when appropriate, were used to compare the two study groups. Simple linear regression analysis was used to investigate the relation between Na+,K+-ATPase activity and ECBA at 3 h of reperfusion. A p < 0.05 was considered significant.
RESULTS
Animal weight, sex, and postnatal age did not differ between groups. Rectal temperature remained stable and within the normal range in all animals. All animals developed severe metabolic acidosis with increased lactate concentrations and were supplemented with NaHCO3 in the immediate posthypoxia-ischemia period. Seizure activity was noted in four and three animals of the PLAC-treated and DFO-treated lambs, respectively. One lamb of the PLAC-treated group died in the immediate posthypoxia-ischemia period and was excluded from the study.
Table 1 shows mean values (± SEM) of mean aortic blood pressure, V˙car, pH, and blood gases at baseline condition, at the end of hypoxia and additional ischemia, and at 3 h after hypoxia-ischemia. Mean aortic blood pressure, pH, and blood gases normalized in all groups at 3 h after hypoxia-ischemia and did not differ between groups during this point. [More detailed information concerning above mentioned variables, including free iron concentrations in blood and cortical brain tissue, were reported previously (4, 6).]
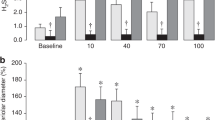
Figure 1A shows the individual Na+,K+-ATPase activity values in cortical brain tissue at 3 h after hypoxia-ischemia in both study groups and in the SHAM animals. Na+,K+-ATPase activity was significantly higher in the DFO-treated lambs compared with the PLAC-treated lambs (means ± SD: 42.0 ± 7.6 versus 35.1 ± 7.4 μmol Pi/mg protein per h;p < 0.05). The two SHAM lambs had similar Na+,K+-ATPase activity values (40.6 and 40.8 μmol Pi/mg protein per h) compared with the DFO-treated lambs.
Box-and-whisker plot and individual values of Na+,K+-ATPase activity of cortical brain tissue (A) and ECBA (B) at 3 h after hypoxia-ischemia in PLAC-treated (open squares) and DFO-treated (black triangles) lambs. As can be seen in A, Na+,K+-ATPase-activity in PLAC-treated but not in DFO-treated lambs tended to be reduced compared with SHAM lambs. (1 indicates the single high values of Na+,K+-ATPase activity and ECBA of a PLAC-treated lamb.)
Figure 1B shows the individual ECBA values at 3 h after hypoxia-ischemia (means ± SEM) of both study groups and the SHAM animals. Although a substantial difference was found (means ± SD: 11.2 ± 6.1 versus 14.8 ± 4.8 μV) the difference was not significant (p = 0.06). However, when the single high value of 25 μV was omitted from one of the PLAC-treated lambs, the difference was highly significant [DFO-treated lambs versus PLAC-treated lambs (means ± SD): 14.8 ± 4.8 versus 9.0 ± 1.0 μV, p < 0.01]. ECBA in the two SHAM lambs were 16.0 and 18.0 μV, respectively. ECBA values at baseline condition did not differ between groups and were in the normal range (means ± SD: 16.7 ± 9.3 for PLAC-treated lambs and 13.7 ± 4.3 μV for DFO-treated lambs). At the end of hypoxia and additional ischemia, both PLAC-treated and DFO-treated lambs showed significantly lower ECBA values compared with baseline (means ± SD: PLAC-treated lambs, 3.5 ± 2.4 μV; DFO-treated lambs, 5.8 ± 4.2 μV;p < 0.0001 versus baseline condition). At 3 h after hypoxia-ischemia, PLAC-treated lambs showed significantly lower ECBA values compared with baseline (p < 0.01). This was not the case in the DFO-treated lambs.
The individual values of Na+,K+-ATPase activity and simultaneously measured ECBA in the animals suffering from convulsions were not different from those values measured in nonconvulsive lambs. This was true in PLAC-treated as well as in DFO-treated lambs.
Figure 2 shows the relation between Na+,K+-ATPase activity and simultaneously measured ECBA of the DFO-treated, the PLAC-treated, and the SHAM lambs. There was a statistically significant correlation between Na+,K+-ATPase activity and ECBA (all, r = 0.86, p < 0.0001; PLAC-treated lambs, r = 0.81, p < 0.02; DFO-treated lambs, r = 0.92, p < 0.001). To illustrate that the regression analysis was not heavily weighted by the high Na+,K+-ATPase activity and ECBA values of the single PLAC-treated lamb with high Na+,K+-ATPase activity and ECBA values (see also Fig. 1), we repeated the linear regression analysis without this animal. We found a similar regression coefficient (r = 0.85, p < 0.0001), strongly suggesting that a solid relationship exists between Na+,K+-ATPase activity of cortical neuronal cell membranes and ECBA.
Individual values of Na+,K+-ATPase activity of cortical brain tissue as a function of ECBA at 3 h after hypoxia-ischemia in PLAC-treated (open squares), DFO-treated (black triangles), and SHAM (open circles) lambs. The line represents the regression line of the of Na+,K+-ATPase activity–ECBA relationship between DFO-treated, PLAC-treated, and SHAM lambs (all, r = 0.86, p < 0.0001; PLAC-treated lambs, r = 0.81, p < 0.02; DFO-treated lambs, r = 0.92, p < 0.001). (1 indicates the high value of a single PLAC-treated lamb.)
DISCUSSION
In the present study, significantly higher Na+,K+-ATPase activity in cerebral cortical tissue was found in iron chelator DFO-treated than in PLAC-treated newborn lambs during the early reperfusion stage after a severe hypoxic-ischemic insult. This suggests that immediate free iron chelation has a beneficial effect on the ATP-dependent Na+,K+-pump of the cortical brain cell membrane in the early posthypoxia-ischemia period. Although Na+,K+-ATPase activity of cortical brain cells of normoxic newborn lambs have not been established previously, and values in normoxic newborn piglets are slightly higher [50.9 ± 2.8 Pi/mg protein per hour, (8)] the values in the DFO-treated animals were comparable to the values of this enzyme measured in the two SHAM normoxic lambs.
An increasingly recognized important pathway for free radical formation is hydroxyl production via the free or nonprotein-bound iron-catalyzed Fenton or Haber-Weiss reaction (16). DFO, a free iron chelator, indeed showed the ability to ameliorate neuronal damage induced by hypoxia-ischemia in the newborn dog, if administered in the immediate posthypoxia-ischemia period (17). Most but not all animals of the present study participated also in a study in which we investigated the early posthypoxic-ischemic patterns of brain perfusion, oxygen metabolism, and electrical brain activity. It appeared that these variables recovered to prehypoxia-ischemia values in the animals treated with DFO, which was not the case in those animals treated with a placebo (4). Furthermore, it was previously reported that free iron concentrations in cortical brain tissue of the DFO-treated animals were significantly lower compared with the PLAC-treated animals, demonstrating that DFO rapidly passes the blood–brain barrier to chelate iron (6): Also in the present study, consisting of partly different animals, cortical neuronal free iron concentrations were lower in DFO-treated lambs compared with PLAC-treated lambs [mean ± SD: 2.81 ± 0.88 (n = 5) versus 3.92 ± 0.58 nmol/g tissue (n = 6), p < 0.05]. The finding in the present study that the Na+,K+-ATPase activity was higher in the DFO-treated animals compared with the PLAC-treated lambs further supports the hypothesis that free iron chelation reduces hypoxia-related damage of the immature brain. Although we did not measure membrane lipid peroxidation in the present study, there is ample evidence that Na+,K+-ATPase activity is affected by hypoxia-induced lipid peroxidation of brain cell membranes, especially of synaptosomes (13, 18–20). Therefore, we suggest that lipid peroxidation is responsible for the changes in Na+,K+-ATPase activity observed after hypoxia-ischemia and reperfusion. In addition to lipid peroxidation, hypoxia-induced activation of protein kinase C may reduce Na+,K+-ATPase activity through phosphorylation of the catalytic subunit (21).
An important reason for making the early observation, at 3 h after completion of the hypoxia-ischemia insult, was the knowledge that formation of excess reactive oxygen species such as superoxide and hydrogen peroxide occurs early [0 to 4 h after reperfusion and reoxygenation, (22, 23)] and contributes substantially to posthypoxic-ischemic reperfusion injury of the immature brain (24, 25). Moreover, a recent study in severely asphyxiated newborn babies showed a relationship between adverse outcome and the concentration of free iron in plasma of these babies determined within the first 8 h of life (26), strongly suggesting that liberation of this transition metal in the early reperfusion phase after severe hypoxia and ischemia adds significantly to free radical-induced brain damage. On the other hand, a study in neonatal piglets by Lorek et al. (27), using 31P-MRS, in which the posthypoxia-ischemia period was investigated for 48 h showed a delayed (secondary) cerebral energy failure, which was reported to start 6 to 12 h after the hypoxic-ischemic insult. This MRS-investigated pattern of cerebral energy metabolism was consistently related to gross histologic brain tissue damage in these animals. So, we cannot be entirely sure that Na+,K+-ATPase activity in the DFO-treated lambs did not deteriorate later in the course of the posthypoxia-ischemia period.
Na+,K+-ATPase is a key enzyme in generating membrane potentials and ECBA. Recoveries of ATP synthesis (28), Na+,K+-ATPase activity, and ECBA at 2 h of reoxygenation have been described to occur simultaneously in animal models of perinatal brain hypoxia-ischemia. Recovery of Na+,K+-ATPase activity coincided with restoration of cerebral edema after brain hypoxia-ischemia (29, 30). The severity of decreases in Na+,K+-ATPase activity are dependent on decreases in phosphocreatine as has been measured using 31P-MRS (8). Also, in the present study, the strong correlation between Na+,K+-ATPase activity and ECBA suggests that electrical stability of the cortical brain cell membrane is directly related to electrical brain activity. The significantly higher Na+,K+-ATPase activity together with the fact that electrical brain activity in the DFO-treated lambs was not different at 3 h after hypoxia-ischemia compared with baseline electrical brain activity (unlike electrical brain activity in PLAC-treated lambs) suggests that free iron chelation prevents early failure of the ATP-dependent Na+,K+-pump of the neuronal cell membrane. Further studies are necessary to demonstrate whether these effects are sustained during the so-called secondary energy failure. Because posthypoxia-ischemia reperfusion injury has also been characterized by cerebral hypoperfusion and reduced oxygen consumption and electrical brain activity in the early reperfusion phase, as indicated by several experimental (17, 31, 32) and clinical studies (5, 33), the preservation of brain perfusion, cerebral oxygen metabolism, and electrical brain activity in the early reperfusion and reoxygenation period in DFO-treated lambs compared with PLAC-treated animals (4) further suggest that secondary cerebral energy failure will be prevented. A final remark should be made with regard to the possibility of whether DFO itself, even in this low dose, can affect the Na+,K+-ATPase activity during baseline conditions by chelating free iron or by interacting with the cell membrane or the enzyme. However, we did not find differences between DFO-treated (posthypoxia-ischemia) lambs and the two SHAM (nonhypoxia-ischemia) lambs, suggesting that such a direct effect of DFO is less probable.
In summary, the present study showed that DFO treatment on completion of hypoxia and additional ischemia in the newborn lamb preserved Na+,K+-ATPase activity in cerebral cortical tissue, suggesting reduction of neuronal cell damage by reactive oxygen species liberated in the early reperfusion phase. Furthermore, a positive relationship was detected between Na+,K+-ATPase activity of the cortical brain cell and ECBA. Further studies are necessary to elucidate whether this intervention also prevents the delayed cerebral energy failure after severe hypoxia and ischemia in the newborn lamb.
Abbreviations
- DFO:
-
deferoxamine
- ECBA:
-
electrocortical brain activity
- PLAC:
-
placebo
- V˙car:
-
carotid blood flow
- SHAM:
-
sham-operated animals
- MRS:
-
magnetic resonance spectroscopy
- Pi:
-
inorganic phosphate
References
Saugstad OD, Aasen AO 1986 Plasma hypoxanthine concentrations in pigs: a prognostic aid in hypoxia. Eur Surg Res 12: 123–129
McCord JM 1985 Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med 312: 159–163
Halliwell B 1992 Reactive oxygen species and central nervous system. J Neurochem 59: 1609–1623
Shadid M, Moison RMW, Steendijk P, Hiltermann L, Berger HM, van Bel F 1998 The effect of antioxidative combination therapy on post hypoxic-ischemic perfusion, metabolism, and electrical activity of the newborn brain. Pediatr Res 44: 119–124
Van Bel F, Shadid M, Moison RMW, Dorrepaal CA, Fontijn J, Monteiro L, Van de Bor M, Berger HM 1998 Effect of allopurinol on postasphyxial free radical formation, cerebral hemodynamics and electrical brain activity. Pediatrics 101: 185–193
Shadid M, Buonocore G, Groenendaal F, Moison RWM, Ferrali M, Berger HM, Van Bel F 1998 Effect of deferoxamine and allopurinol on non-protein-bound iron concentrations in plasma and cortical brain tissue of newborn lambs following hypoxia-ischemia. Neurosci Lett 248: 5–8
Mishra OP, Delivoria-Papadopoulos M 1988 Na+,K+-ATPase in developing fetal guinea pig brain and the effect of maternal hypoxia. Neurochem Res 13: 765–770
DiGiacomo JE, Pane CR, Gwiazdowski S, Mishra OP, Delivoria-Papadopoulos M 1992 Effect of graded hypoxia on brain cell membrane injury in newborn piglets. Biol Neonate 61: 25–32
Dobsen AD, Sellers AF, McLeod FD 1986 Performance of a cuff-type blood flowmeter in vivo. J Appl Physiol 21: 1642–1648
Van Bel F, Roman C, Klautz RJM, Teitel DF, Rudolph AM 1994 Relationship between brain blood flow and carotid arterial flow in the sheep fetus. Pediatr Res 35: 329–333
Prior PF 1979 Monitoring Cerebral Function: Long-Term Recordings of Cerebral Electrical Activity. North Holland Biomedical Press, Amsterdam, pp 45–301
Hellstrom-Westas L, Rosen I, Svenningsen HW 1995 Predictive value of early continuous amplitude integrated EEG recordings on outcome after severe birth asphyxia in full term infants. Arch Dis Child 72: F34
Harik SI, Doull GH, Dick APK 1985 Specific ouabain binding to brain microvessels and choroid plexus. J Cereb Blood Flow Metab 5: 156–160
Mishra OP, Delivoria-Papadopoulos M, Cahillane G, Wagerle LC 1989 Lipid peroxidation as the mechanism of modification of the affinity of the Na+,K+-ATPase active sites for ATP, K+, Na+ and strophanthin in vitro. Neurochem Res 14: 845–851
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ 1951 Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275
Halliwell B, Chirico S 1993 Lipid peroxidation: its mechanism, measurements and significance. Am J Clin Nutr 57: 715S–725S
Hurn PD, Koehler RC, Blizzard KK, Traystman RJ 1995 Deferoxamine reduces early metabolic failure associated with severe cerebral ischemic acidosis in dogs. Stroke 26: 688–695
Mishra OP, Delivoria-Papadopoulos M, Cahillane G, Wagerle LC 1990 Lipid peroxidation as the mechanism of modification of brain 5′-nucleotidase activity in vitro. Neurochem Res 15: 237–242
Razdan B, Marro PJ, Tammela O, Goel R, Mishra OP, Delivoria-Papadopoulos M 1993 Selective sensitivity of synaptosomal membrane function to cerebral cortical hypoxia in newborn piglets. Brain Res 600: 308–314
Kovachich GB, Mishra OP 1981 Partial inactivation of Na+,K+-ATPase in cortical brain slices incubated in normal Krebs-Ringer phosphate medium at 1 and 10 atm oxygen pressures. J Neurochem 36: 333–335
Bertorello AM, Aperia A, Walaas SI, Nairn AC, Greengard P 1991 Phosphorylation of the catalytic subunit of Na+,K+-ATPase inhibits the activity of the enzyme. Proc Natl Acad Sci USA 88: 11359–11362
Siesjo BK, Siesjo P 1996 Mechanisms of secondary brain injury. Eur J Anaesthesiol 13: 247–268
Hayashi T, Sakurai M, Itoyma Y, Abe K 1999 Oxidative damage and breakage of DNA in rat brain after transient MCA occlusion. Brain Res 832: 159–163
Saugstad OD 1990 Oxygen toxicity in the neonatal period. Acta Paediatr 79: 881–892
Traystman RJ, Kirch JR, Koehler RC 1991 Oxygen radical mechanisms of brain injury following ischemia and reperfusion. J Appl Physiol 71: 1185–1195
Dorrepaal CA, Berger HM, Benders MJNL, Van Zoeren-Grobben D, Van De Bor M, Van Bel F 1996 Non protein bound iron in postasphyxial reperfusion injury of the newborn. Pediatrics 98: 883–889
Lorek A, Takei Y, Cady EB, Wyatt JS, Penrice J, Edwards AD, Peebles D, Wylezinska M, Owen-Reece H, Kirkbride V, Cooper CE, Aldridge RF, Roth SC, Brown G, Delpy DT, Reynolds EOR 1994 Delayed (“secondary”) cerebral energy failure after acute hypoxia-ischemia in the newborn piglet: continuous 48-hour studies by phosphorus magnetic resonance spectroscopy. Pediatr Res 36: 699–706
Groenendaal F, de Graaf RA, van Vliet G, Nicolay K 1999 Effects of hypoxia-ischemia and inhibition of nitric oxide synthase on cerebral energy metabolism in newborn piglets. Pediatr Res 45: 827–833
Mintorovitch J, Yang GY, Shimuzu H, Kucharczyk J, Chan PH, Weinstein PR 1994 Diffusion-weighted magnetic resonance imaging of acute focal cerebral ischemia: comparison of signal intensity with changes in brain water and Na+,K+-ATPase activity. J Cereb Blood Flow Metab 14: 332–336
Allen KL, Busza AL, Proctor E, Williams SR, Van Bruggen N, Gadian DG, Crockard HA 1990 Restoration of energy metabolism and resolution of oedema following profound ischaemia. Acta Neurochir Suppl Wien 51: 171–173
Williams CE, Gunn AJ, Mallard EC, Gluckman PD 1991 Outcome after ischemia in the developing sheep brain: an electro-encephalographic and histological study. Ann Neurol 31: 14–21
Hossmann KA 1993 Ischemia mediated neuronal injury. Resuscitation 26: 225–235
Van Bel F, Dorrepaal CA, Benders MJNL, Zeeuw PEM, Van De Bor M, Berger HM 1993 Changes in cerebral hemodynamics and oxygenation in the first 24 hours after birth asphyxia. Pediatrics 92: 365–372
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Groenendaal, F., Shadid, M., McGowan, J. et al. Effects of Deferoxamine, a Chelator of Free Iron, on Na+,K+-ATPase Activity of Cortical Brain Cell Membrane during Early Reperfusion after Hypoxia-Ischemia in Newborn Lambs. Pediatr Res 48, 560–564 (2000). https://doi.org/10.1203/00006450-200010000-00023
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200010000-00023
This article is cited by
-
Ischemia-reperfusion injury: molecular mechanisms and therapeutic targets
Signal Transduction and Targeted Therapy (2024)
-
Prophylactic inhibition of NF-κB expression in microglia leads to attenuation of hypoxic ischemic injury of the immature brain
Journal of Neuroinflammation (2020)
-
Iron overload impact on P-ATPases
Annals of Hematology (2018)
-
Critical Evaluation of the Changes in Glutamine Synthetase Activity in Models of Cerebral Stroke
Neurochemical Research (2015)
-
Iron metabolism and lipid peroxidation products in infants with hypoxic ischemic encephalopathy
Journal of Perinatology (2008)