Abstract
C57/B6 mice received intraperitoneal horse spleen apoferritin (4 mg) with lipopolysaccharide (0.05 mg); control mice received 0.15 M NaCl. Control and treated animals were killed weekly for 6 wk; blood and urine specimens were obtained, and tissue samples were secured. Treated animals showed evidence of significant chronic disease, with proteinuria, hematuria, and uremia. A mild glomerulonephritis was present at 2 wk, with significant proliferative glomerulonephritis at 4 wk, progressing to chronic disease with tubulointerstitial changes at 6 wk. Changes at each time period were uniform between animals. C3 mRNA was first detected by in situ hybridization at 3 wk. Message was restricted to proximal tubular and periglomerular epithelial cells. Presence of C3 message preceded the development of interstitial inflammation and fibrosis by 1–2 wk, and its location and intensity paralleled the evolving interstitial disease. Although extensive mesangial C3 protein deposits appeared early, there was never C3 message in glomeruli or infiltrating cells. Before C3 message became apparent, two cytokines known to up-regulate C3 transcription in vitro, IL-1 and IL-6, were detected by immunohistochemistry. The temporal sequence in this model is consistent with our hypothesis that local synthesis and activation of C3 in tubular epithelium is important to the interstitial component of chronic glomerulonephritis. The process is independent of the deposition of circulating complement in the glomerulus, but may be triggered by glomerular cytokines.
Similar content being viewed by others
Main
A variety of primary glomerular diseases can progress to chronic renal failure. Common to all of these is involvement of the interstitium with peritubular inflammation, edema, fibrosis, and tubular atrophy (1). Progression to end-stage renal disease never occurs in the absence of these features, and their presence in the early stage of disease is often predictive of deterioration (2).
Despite recognition of this sequence, there is little understanding of the mechanisms by which glomerular inflammation triggers an interstitial process. Studies of human biopsy material from our laboratory have suggested a role for local expression of complement by the tubular epithelium (3–5). A limitation of this work has been the fact that such material represents a single time point in the evolution of a complex disease. Thus, it is not possible to place the presence of tubular complement component expression into a temporal context.
In an effort to test further the hypothesis that tubular complement expression was a potential mediating factor in the progression of glomerulonephritis, we determined that an animal model would be necessary. The model would need to feature tubular complement expression, and involve a species with a defined complement system. It should begin with acute glomerular inflammation, with gradual progression to interstitial involvement, permitting assessment of the time at which local complement synthesis began. The studies reported here describe the features of such a model, and the evidence supporting a role for local expression of complement component C3.
METHODS
Animals.
Six-week-old male C57/B6 mice were obtained from the Jackson Laboratory (Bar Harbor, ME, U.S.A.). The study was approved by the Animal Use Committee of the Children's Hospital Research Foundation.
Antisera and reagents.
FITC-conjugated goat anti-murine C3c (the major fragment of C3; Nordic, Capistrano Beach, CA, U.S.A.), rabbit anti-mouse IL-1∝ (Endogen, Cambridge, MA, U.S.A.) rabbit anti-mouse IL-6 (Genzyme, Cambridge, MA, U.S.A.), FITC-conjugated goat anti-rabbit IgG (Rockland, Gilbertsville, PA, U.S.A.), HSA (Sigma Chemical Co., St. Louis, MO, U.S.A.), and LPS (Calbiochem, San Diego, CA, U.S.A.) were obtained as indicated.
Riboprobes.
The plasmid pBR322 containing a 2.4-kb mouse cDNA insert for C3 was obtained from American Type Culture Collection (Rockville, MD, U.S.A.). A 541-bp product was generated by PCR using a 5′ CAA GGC TTG CAA TAC CAT GAA GG 3′ and a 5′ CCT GTG TGG GGC TGT TAA ATG 3′ oligonucleotide. The PCR product was then ligated into a PCR 2.1 TA cloning vector (Invitrogen, Carlsbad, CA, U.S.A.). Colonies were grown and checked by restriction enzyme digestion for orientation to obtain sense and antisense probes. DNA was isolated using Qiagen purification (Santa Clarita, CA, U.S.A.), linearized, and then transcribed using T7 polymerase, 35S-UTP, and a Promega (Madison, WI, U.S.A.) transcription kit. The RNA probes were passed over quick-spin columns (Boehringer Mannheim, Indianapolis, IN, U.S.A.) to remove unincorporated nucleotides before use.
Urine and blood samples.
Blood urea nitrogen concentration was measured by the Children's Hospital Medical Center clinical laboratory. The assay used (Vitros 950, Johnson & Johnson, Rochester, NY, U.S.A.) has a reported coefficient of variation of 1.6%. A normal range for murine blood urea concentration of 12–22 mg/dL was established in 10 normal 6-week-old animals. Urine was analyzed for the presence of blood and protein by Multistix (Bayer Corporation, Elkhart, IN, U.S.A.), according to directions supplied by the manufacturer.
Tissue harvesting and processing.
Animals were killed by CO2 narcosis. Immediately after death, the kidneys were dissected free and bisected. Tissue samples were frozen in M-1 embedding matrix (Shandon Lipshaw, Pittsburgh, PA, U.S.A.) for immunohistochemistry or treated with 10% formalin for routine histology or 4% paraformaldehyde for in situ hybridization.
Blood samples were obtained at the time of death by cardiac puncture. Before killing, animals were held in metabolic cages to obtain urine specimens.
Immunohistochemistry.
Frozen tissue was cut into 10-μm sections, dried on positively charged slides, washed with PBS, and incubated at 37°C for 30 min with 10% normal goat serum. Excess serum was blotted, and the slides were incubated with a 1/50 dilution of primary antibody for 1 h at 37°C. The tissue was washed with PBS. The slides with IL-1∝ and IL-6 antibodies were then incubated with FITC-conjugated goat anti-rabbit IgG (1/20 dilution) for 30 min at 37°C and washed again with PBS. All slides were then mounted with 0.1%p-phenylenediamine in PBS-glycerol and coded before examination. Treated sections were examined with UV light in an incident-light fluorescent microscope, and the fluorescence pattern (focal, diffuse; mesangial, loop) was defined. Fluorescence intensity was graded as negative, or 1+ through 4+. Control sections lacking primary antibody were included with each experiment. All sections were examined by two authors (T.R.W. and D.W.) independently.
Histology.
Paraffin-fixed tissue was cut into 4- to 6-μm sections and stained with H&E, PAS, and Jones silver-methenamine by standard methods. Sections were coded and examined independently by two of the authors (T.R.W. and D.W.) using a variation of the method we have described previously (6). Glomerular and interstitial regions were each scored. Glomeruli were scored on a scale of a 0 (absent) to 4+ (extensive, involving all glomeruli) for mesangial proliferation and mesangial matrix increase. The interstitium was similarly scored for peritubular inflammation, peritubular matrix increase, and tubular atrophy.
In situ hybridization.
Paraformaldehyde-fixed tissue was used for C3 in situ hybridization, as previously described from our laboratory (3–5). Sense strand riboprobes were included in all experiments as negative controls, and mouse liver was used as a positive tissue control.
Sections were examined by dark-field microscopy at ×20 and ×40. The presence, distribution, and intensity of signal was recorded for each tissue by two of the authors (T.R.W. and D.W.). Signal that was not different from sense strand control (such as Fig. 1F) was graded as 0, whereas occasional scattered foci were graded 1+. Focal tubular label involving ≤50% of control tubules (such as Fig. 1G) was graded 2+. Label involving >50% of tubules was 3+, whereas >75% (Fig. 1H) constituted a 4+ label. A similar scale was applied to periglomerular (Bowman's capsule) label.
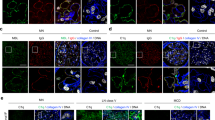
A, H&E-stained section from saline-injected animal at 4 wk to illustrate normal glomerulus and interstitium (×450);B, H&E-stained section from HSA/LPS-injected animal at 4 wk, illustrating proliferative glomerulonephritis (gl) and early periglomerular and peritubular infiltrate (arrow) (×450);C, PAS-stained section from HSA/LPS-treated animal at 6 wk, illustrating extensive, advanced interstitial involvement (×450);D, ∝C3c-stained section from HSA/LPS-treated animal at 2 wk, illustrating early presence of complement deposits in glomerular mesangium (gl) (×500);E, IL-1∝-stained section from HSA/LPS-treated animal at 4 wk, illustrating presence of cytokine in a mesangial distribution (gl) (×500);F, C3 in situ hybridization of section from HSA/LPS-treated animal at 1 wk, illustrating absence of signal early (×100);G, C3 in situ hybridization of section from HSA/LPS-treated animal at 4 wk illustrating focal signal in a tubular and periglomerular distribution (×100), H, C3 in situ hybridization of section from HSA/LPS-treated animal at 5 wk, illustrating extensive, generalized tubular and periglomerular signal (×100).
Experimental protocol.
Animals received injections of HSA (4 mg) intraperitoneally five times weekly, with LPS (0.05 mg) intraperitoneally three times weekly. Control animals received 0.15 M NaCl (0.1 mL) five times weekly. At intervals of 1 wk for a total of 6 wk, one saline and three HSA/LPS-treated animals were killed, with blood, urine, and tissue samples obtained as described.
This model was adapted from a previously described method (7). In pilot studies undertaken to determine the optimal immunization schedule, we determined that extending the HSA immunization from the original 14 d and adding LPS as an immune adjuvant produced the most consistent, reproducible lesion.
RESULTS
Histology
Control animals.
The appearance of the saline-injected control animals was uniform at each weekly interval. No changes in mesangial cellularity or matrix or in the morphology of the tubulointerstitial areas were seen (Fig. 1A).
Treated animals.
Between wk 1 and 3, there was a uniform, progressive increase in mesangial matrix and cellularity. No interstitial lesions were present. At 4 wk, the glomerular lesions were more advanced, and rare foci of periglomerular and peritubular mononuclear infiltration were present (Fig. 1B). At wk 5, the glomerular changes included some obliteration, which involved half the glomeruli by wk 6. The tubular changes also progressed, with widespread infiltrate and occasional atrophy at wk 5, followed by extensive fibrosis, and atrophy at wk 6 (Fig. 1C). Most sections at wk 6 showed essentially end-stage renal disease.
Immunohistochemistry
At wk 1, there was no fluorescence with any antisera. Coarse granular deposits (1+) of C3c first appeared at wk 2 (Fig. 1D), and progressed to a generalized mesangial pattern that was well established (2+) by wk 3 and continued through wk 6 (3+). Beginning at wk 4, some tubular C3c protein was also present.
The IL-1∝ and IL-6 patterns were similar. Generalized trace reaction for both cytokines in a mesangial distribution (1+) was first detected at 2 wk. This was well-established by 3–4 wk (2+), and continued unchanged through the end of the study (Fig. 1E).
C3 mRNA by in situ hybridization
Control hybridization of the sense and antisense C3 riboprobe on murine liver confirmed its activity and specificity. No C3 mRNA was seen in the kidney tissue of saline-injected mice at any time.
The treated animals showed no C3 mRNA on wk 1 or 2 (Fig. 1F). By wk 3, however, definite foci of signal were detected in a periglomerular and proximal tubular location (1+). By 4 wk, this activity was more extensive (2+), and the signal was stronger (Fig. 1G). Progressive increase in signal intensity and distribution occurred on wk 5 (3+) and 6 (4+) (Fig. 1H).
With the exception of the periglomerular signal (from the epithelium of Bowman's capsule), there was no evidence of glomerular C3 mRNA at any time. This observation was maintained even in situations in which the histology demonstrated an extensive glomerular infiltrate and immunohistochemistry showed extensive C3 protein deposits.
Also, the interstitial signal was restricted to proximal tubular epithelial cells. At no time was there evidence of message in infiltrating inflammatory cells or in blood vessels. Low-magnification scanning of sections never showed signal in the medullary portions of the kidney.
Clinical disease
The animals had hematuria, proteinuria, and progressive renal insufficiency (elevated urea nitrogen), as detailed in Fig. 2. Two treated animals died, one each at 2 and 3 wk.
Summary of clinical, histologic, immunohistochemical, and in situ hybridization findings in treated animals. The degree of shading in the bars represents the 0 to 4+ scale used in tissue analysis (see text for details). The scales for urine blood (trace to large) and protein (trace to 4+) are based on the dipstick method used.
Integration of findings
In Figure 2, the sequence of evolution of these findings is summarized. The depth of shading in this figure represents the 0–4+ scales described in “Methods.” It is notable that glomerular cytokine activity is established by wk 2, before any C3 mRNA is seen. In turn, development of interstitial C3 message at 3–4 wk clearly antedates the development of tubular injury, which is rare and spotty at wk 4 and not established until wk 5. Finally, the most dramatic change in clinical findings (urinalysis and blood urea nitrogen) do not begin until tubulointerstitial injury is established.
DISCUSSION
Most reported models of glomerulonephritis concentrate on the development and evolution of the glomerular lesion. In human disease, however, the severity of glomerulonephritis is nearly always marked by the nature and extent of the tubulointerstitial involvement. In systemic lupus erythematosus, for example, Esdaile et al. (8) have shown that the factor most predictive of deterioration in renal function is the presence of interstitial involvement in the initial kidney biopsy. Similar observations have been made in IgA nephropathy (9, 10) and membranoproliferative glomerulonephritis (11). Tubulointerstitial injury per se results in deterioration in renal function (1, 12).
The mechanism by which some glomerular inflammatory processes lead to tubulointerstitial disease are not well understood. Such an understanding, however, is potentially very important. First, it might allow the early recognition of disorders at high risk of progression. Second, it might permit the design of new treatment strategies.
With human biopsy tissue studies, we have suggested a role for tubular complement synthesis in this process. Although complement component C4 appears to be expressed constitutively in all human kidneys studied (3), C3 (4) and B (5) are differentially regulated.
By in situ hybridization, message for these two components of the alternative pathway C3 convertase are expressed in the proximal tubular epithelium of kidneys with interstitial disease. In the case of C3, this observation has been confirmed by others (13). Although one group has reported C3 expression by glomerular cells as well (14), the equal intensity of tubular message in this study, in our opinion, makes the observation questionable. Even if the low-level glomerular expression in this report were real, tubular expression should have been proportionately much more extensive. In any case, it is difficult to ascribe importance to low levels of locally produced complement in a structure (the glomerulus) continuously bathed with plasma components.
Evidence of human renal C3 synthesis has led many to suggest a role for local complement in disease. For the most part, however, these suggestions have related to a role in glomerular inflammation (15). Our work, however, has led to the generation of a hypothesis that relates local complement synthesis to progressive interstitial injury. This hypothesis is based both on our work and on that of others, and is the basis for the studies described here. According to this hypothesis, the initial insult in most immune glomerulonephritides includes direct complement-mediated glomerular injury. The complement source for this initial step is the circulation (16).
In the course of this glomerular injury, a variety of cytokines are expressed in the mesangium, either synthesized by resident mesangial cells or by infiltrating inflammatory cells (17, 18). Several of these cytokines (IL-1 and IL-6, for example), have been shown to up-regulate C3 transcription (19). We suggest that these cytokines may gain access to the proximal tubular epithelium and the epithelium of Bowman's capsule via glomerular filtrate or, in the case of the proximal tubule, the vasa recta. This, in turn, could up-regulate C3 expression in these cells.
Once C3 protein is present in the interstitium, there are a variety of ways in which it could be activated. Local cell injury, for example, could result in the basement membrane of some cells becoming alternative pathway activators. In one in vitro study (20), proximal tubular epithelial cells in culture were shown to be alternative pathway activators. The ammonia content of the interstitium (21, 22) may also result in the formation of NH3·C3, an effective convertase.
In any event, local production and activation of C3 would then be capable of mediating interstitial effects in a variety of ways. Nonlytic insertion of the MAC into the cell could result in expression of a variety of proteins, which, in turn, could mediate progressive inflammation, fibrosis, or cell death (23). Occupation of C5a receptors could have similar results. Fluid-phase anaphylatoxins could also be generated by this process, further recruiting inflammatory cells to the peritubular environment.
Both our human biopsy studies (3–5) and the model described herein are consistent with this hypothesis. These data suggest that the role of complement in glomerulonephritis may include, in addition to the well-established induction of glomerular injury, mediating the chronic interstitial injury that accompanies progressive disease. The availability of this well-characterized model will now permit a more precise study of the role of complement with gene targeting, as well as analysis of genes induced in tubular cells by complement deposition. Such studies, currently ongoing, should also make it possible to define the complement dependence of the model.
Abbreviations
- LPS:
-
lipopolysaccharide
- HSA:
-
horse spleen apoferritin
- PAS:
-
periodic acid–Schiff
- H&E:
-
hematoxylin and eosin
- MAC:
-
membrane attack complex
References
Mackensen-Haen S, Bohle A, Christensen J, Wehrmann M, Kendziorra H, Fokot F 1992 The consequences for renal function of widening of the interstitium and changes in the tubular epithelium of the renal cortex and outer medulla in various renal diseases. Clin Nephrol 37: 70–77.
Bohle A, Mackensen-Haen S, von Gise H, Grund KE, Wehrmann M, Batz C, Bogenschultz O, Schmitt H, Nagy J, Muller C 1990 The consequences of tubulointerstitial changes for renal function in glomerulonephritis. Pathol Res Pract 186: 135–144.
Witte DP, Welch TR, Beischel LS 1991 Detection and cellular localization of human C4 gene expression in the renal tubular epithelial cells and other extrahepatic epithelial sources. Am J Pathol 139: 717–724.
Welch TR, Beischel LS, Witte DP 1993 Differential expression of complement C3 and C4 in the human kidney. J Clin Invest 92: 1451–1458.
Welch TR, Beischel LS, Frenzke M, Witte D 1996 Regulated expression of complement factor B in the human kidney. Kidney Int 50: 521–525.
Welch TR, Fryer C, Shely E, Witte DP, Quinlan M 1992 Double-blind, controlled trial of short-term prednisone therapy in immunoglobulin A glomerulonephritis. J Pediatr 121: 474–477.
Iskandar SS, Gifford DR, Emancipator SN 1988 Immune complex acute necrotizing glomerulonephritis with progression to diffuse glomerulosclerosis. Lab Invest 59: 772–779.
Esdaile JM, Levinton C, Federgreen W, Hayslett JP, Kashgarian MK 1989 The clinical and renal biopsy predictors of long-term outcome in lupus nephritis: a study of 87 patients and review of the literature. Q J Med 269: 779–833.
Bogenschutz O, Bohle A, Batz C, Wehrmann M, Pressler H, Kendziorra H, Gartner HV 1990 IgA nephritis: in the importance of morphological and clinical parameters in the long-term prognosis of 239 patients. Am J Nephrol 10: 137–147.
Lee S-MK, Rao VM, Franklin WA, Schiffer MS, Aronson AJ, Spargo BH, Katz AI 1982 IgA nephropathy: morphologic predictors of progressive renal disease. Hum Pathol 13: 314–322.
Schmitt H, Bohle A, Reineke T, Mayer-Eichberger D, Wolfgang V 1990 Long-term prognosis of membranoproliferative glomerulonephritis type I. Nephron 55: 242–250.
Bohle A, Mackensen-Haen S, von Gise H 1987 Significance of tubulointerstitial changes in the renal cortex for the excretory function and concentration ability of the kidney: a morphometric contribution. Am J Nephrol 7: 421–433.
Montinaro V, Gesualdo L, Ranieri E, Monno R, Grandaliano G, Schena FP 1997 Renal cortical complement C3 gene expression in IgA nephropathy. J Am Soc Nephrol 8: 415–425.
Miyazaki M, Abe K, Koji T, Furusu A, Ozono Y, Harada T, Nakane PK, Yagame M, Endoh M, Nomoto Y 1996 Intraglomerular C3 synthesis in human kidney detected by in situ hybridization. J Am Soc Nephrol 7: 2428–2433.
Sacks SH, Zhou W, Sheerin NS 1996 Complement synthesis in the injured kidney: does it have a role in immune complex glomerulonephritis?. J Am Soc Nephrol 7: 2314–2319.
Hebert LA, Fernando GC, Birmingham DJ 1992 The role of the complement system in renal injury. Semin Nephrol 12: 408–427.
Chen W-P, Lin C-Y 1994 Augmented expression of interleukin-6 and interleukin-1 genes in the mesangium of IgM mesangial nephropathy. Nephron 68: 10–19.
Horii Y, Muraguchi A, Iwano M, Matsuda T, Hirayama T, Yamada H, Fujii Y, Dohi K, Ishikawa H, Ohmoto Y, Yoshizaki K, Hirano T, Kishimoto T 1989 Involvement of IL-6 in mesangial proliferative glomerulonephritis. J Immunol 143: 3949–3955.
Falus A, Rokita H, Walcz E, Brozik M, Hidvegi T, Meretey K 1990 Hormonal regulation of complement bio-synthesis in human cell lines. Mol Immunol 27: 197–201.
Biancone L, David S, Pietra VD, Montrucchio G, Cambi V, Camussi G 1994 Alternative pathway activation of complement by cultured human proximal tubular epithelial cells. Kidney Int 45: 451–460.
Gordon DL, Krueger RA, Quie PG, Hostetter MK 1985 Amidation of C3 at the thiolester site: stimulation of chemiluminescence and phagocytosis by a new inflammatory mediator. J Immunol 134: 3339–3345.
Nath KA, Hostetter MK, Hostetter TH 1989 Ammonia complement interaction in the pathogenesis of progressive renal injury. Kidney Int 36: 52–54.
Morgan BP 1989 Complement membrane attack on nucleated cells: resistance, recovery and non-lethal effects. Biochem J 264: 1–14.
Acknowledgements
The authors thank Allene Ford and Jan Messer for careful secretarial assistance, and Alicia Emley for skilled technical assistance.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Welch, T., Frenzke, M. & Witte, D. Evidence of a Role for Local Complement Expression in a Murine Model of Progressive Glomerulonephritis. Pediatr Res 48, 200–205 (2000). https://doi.org/10.1203/00006450-200008000-00013
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200008000-00013
This article is cited by
-
The Lupus-derived Anti-double-stranded DNA IgG Contributes to Myofibroblast-like Phenotype in Mesangial Cells
Journal of Clinical Immunology (2012)