Abstract
Platelet activating factor (PAF) is an inflammatory lipid mediator released by ischemic brain. Our objectives were to use an inhibitor of PAF that does not readily cross the blood-brain barrier, WEB 2170, to study the role of intravascular PAF on brain swelling and subsequent brain atrophy in a neonatal rat model of hypoxic-ischemic brain injury. We injured the right cerebral hemisphere of 7-d-old rats by ligating the right common carotid artery and exposing the rats to 8% oxygen for 2.25 h. Forty-two rats received saline or the PAF antagonist WEB 2170, 1 h before hypoxia. We found that WEB 2170 pretreatment reduced swelling by 64% (p = 0.003). In contrast, treatment immediately after hypoxic-ischemic injury did not reduce swelling. In two additional experiments involving 103 rats, we found that pretreatment or repeated doses of PAF antagonist before and after hypoxic-ischemic injury did not reduce atrophy. We also found that the brain-penetrating PAF antagonist, BN 52021, did not prevent atrophy in our Wistar rat model. In conclusion, we were unable to reduce long-term brain injury with either PAF antagonist. WEB 2170 pretreatment reduced brain swelling by 64% without reducing atrophy. This suggests that although brain swelling may accompany cerebral infarction, it does not contribute to the pathogenesis of infarction and subsequent atrophy in the neonatal rat. The ability to reduce early postischemic brain swelling without reducing atrophy may be particularly unique to the immature animal with a compliant skull.
Similar content being viewed by others
Main
In all ischemic tissues, including the brain, microvessels bear the brunt of reperfusion injury. PAF contributes to reperfusion injury (1). In the immature, P7 rat model of unilateral carotid artery ligation and systemic hypoxia, the hemisphere ipsilateral to ligation swells progressively for 2 to 3 d from vasogenic edema. Plasma proteins leak through the disrupted blood-brain barrier into the brain parenchyma, and the brain swells as a consequence. There is a direct and linear correlation between the extent of edema and infarct volume in the immature rat model (2).
It is not known to what extent vascular injury and brain swelling contribute to long-term neuropathologic outcome or merely reflect parenchymal damage. This is relevant to cerebral ischemia in newborns, as their compliant skull may protect them from the increase in intracranial pressure that accompanies brain swelling in adults. One way to address this question is to reduce vascular injury and determine whether a reduction in brain swelling is accompanied by a reduction in cerebral atrophy. This aim was achieved while we evaluated the neuroprotective potential of a novel PAF antagonist.
PAF is produced by vascular and nonvascular sources in the brain. Vascular production sites include endothelial cells (3), neutrophils, and platelets (4). PAF exerts powerful inflammatory actions on reperfused blood vessels, eventually producing disruption of the blood-brain barrier and vasogenic edema (5). PAF concentrations may be higher in neonatal animals than in the adult as the neonate possesses low amounts of the acetylhydrolase, which is responsible for PAF degradation (6). Accordingly, the contribution of PAF to injury of the immature rat brain may be more significant than in the adult.
The nonvascular sources of PAF in the brain include neurons and microglia (7, 8). PAF acts via specific PAF receptors (9, 10), and in high concentrations, PAF can kill neurons (11). Studies in adult animal models using specific PAF receptor antagonists show reversal or prevention of the consequences of ischemic brain injury (12, 13). These studies support a role for PAF as an important mediator of ischemic brain injury. The relative contribution of vascularly derived PAF versus PAF that is not derived from the parenchyma is not known. The role of PAF antagonists in neonatal ischemic brain injury is limited to a single study in P7 rats in which Liu et al. (14) reduced cerebral infarction by 60% with BN 52021, a PAF antagonist that crosses the blood-brain barrier quite readily.
In this study, we used a PAF antagonist called WEB 2170 (Boehringer Ingelheim, Ridgefield, CT, U.S.A.). Autoradiographs of radioisotope-labeled WEB 2170 injected into rodents revealed only minor penetration across the blood-brain barrier (Dr. G. Schnorrenberg, Boehringer Ingelheim KG, personal communication, 1996). This novel PAF antagonist reduced the development of brain swelling after concussive trauma to adult rat brain (15). It has not been tested in an animal model of cerebral ischemia.
Our first objective was to determine whether WEB 2170 could reduce post-HI brain swelling. Our second objective was to determine whether WEB 2170 could reduce long-term brain injury as measured by cerebral atrophy. We also wanted to determine whether brain injury could be reduced when WEB 2170 was administered after HI insult.
METHODS
We induced an HI insult to the right cerebral hemisphere of P7 rats. Some animals received treatment before hypoxia-ischemia and others immediately after recovery. Animals were killed after a short (42 h) or long (14 d) recovery period to evaluate the extent of brain swelling or brain atrophy, respectively.
Our Committee on Animal Investigation approved all procedures used in this study.
Animal model
P7 Wistar (Charles River, Wilmington, MA, U.S.A.) rat pups of either sex (weight, 12–16 g), were weighed and anesthetized with a mixture of halothane (3% halothane for induction, 2% for maintenance), 30% oxygen, and balance nitrous oxide. The right common carotid artery of each pup was permanently ligated with 4–0 surgical silk through a midline neck incision. The wound was sutured, and the animals were allowed to recover with their dams for 3 h. The duration of anesthesia was about 5 min. After carotid artery ligation, the pups were numbered sequentially and randomized into treatment groups (16). (Treatment was injected s.c. either 1 h before hypoxia or immediately after hypoxia as discussed below.) Then three or four rat pups (equally representing the treatment groups) were placed in 500-mL airtight jars and exposed to a continuous flow of 8% oxygen–92% nitrogen. The jars were partially submerged in a 37°C water bath to maintain a constant thermal environment. Air temperature in the middle of the jar was maintained between 32.6°C and 33.1°C. After 2.25 h of hypoxia, the jars were opened to room air (0 h recovery), and the pups were returned to recover with their dams. The rats were weighed again before they were killed.
Measurement of weight gain
The weight gain was calculated from the change in weight from the time of ligation and at 42 h of recovery from hypoxia. The gain was expressed as a percentage.
Measurement of brain swelling
After 42 h of recovery with their dams, the pups were decapitated, and their brains were removed. The posterolateral portion (∼150–200 mg) from each cerebral hemisphere was placed in a preweighed 5-mL glass vial and then weighed on a microanalytical balance. The posterolateral portion of the right hemisphere (ipsilateral to common carotid artery ligation) represented the area most severely injured, whereas the corresponding area in the left hemisphere served as control. Subsequently, the specimen was desiccated at 80°C for 48–72 h. Reweighing ascertained the dry weight of the tissue, and by subtraction from the wet weight, the water content of the hemisphere was obtained. Water content and dry weight were determined as a percentage of the wet brain weight. The hemisphere contralateral to carotid artery ligation does not undergo an alteration in water content 42 h after HI injury (17).
Right hemisphere swelling was calculated from the change (reduction) in percentage dry weight of the damaged right hemisphere compared with the undamaged left hemisphere and expressed as a percentage.
Measurement of brain atrophy
At the end of the HI insult, the animals were returned to their dams. Cutting a small notch in the right ear identified treatment groups. This procedure incurred minimal bleeding. At 14 d of recovery, the animals were anesthetized with 150 mg of i.p. pentobarbital and decapitated. The brains were carefully removed from the skull, the cerebellum was removed, and the hemispheres were then divided sagittally and immediately weighed. The difference in hemispheric weight was determined as a percentage of the weight of the undamaged hemisphere and expressed as the percentage hemisphere weight deficit (atrophy) (18).
Neuroprotection experiments
Experiment 1: Evaluation of brain swelling with WEB 2170pretreatment.
To determine whether WEB 2170 was neuroprotective when administered 1 h before the cerebral HI insult, we randomized 42 P7 rats into two treatment groups at the time of right carotid artery ligation. After 2 h recovery from ligation (1 h before HI), the rats were treated with an s.c. injection of either WEB 2170, which had been freshly dissolved in sterile water, or an equal volume of saline solution (0.01 mL/g). The pups were returned to their dams for 1 h before being subjected to hypoxia for 2.25 h. At 42 h recovery, the rats were killed, and right hemisphere swelling was calculated. We elected to use the 10-mg/kg dose, as other investigators had shown it to be effective in reducing tissue injury (19, 20).
Experiment 2: Evaluation of brain swelling with WEB 2170posttreatment.
In a separate experiment, 45 rat pups were randomized at the time of ligation into three groups. After 3 h recovery from ligation, the pups were subjected to hypoxia. Immediately upon recovery from HI, rat pups were treated with WEB 2170 as a single s.c. injection of 1 or 10 mg/kg, or an equal volume of saline solution. Animals were killed at 42 h recovery after HI for determination of right hemisphere swelling.
Experiment 3: Evaluation of brain atrophy with WEB 2170pretreatment.
To determine whether pretreatment with WEB 2170 produces long-term neuroprotection from HI, we randomized 59 rat pups into two treatment groups at the time of right carotid artery ligation. Pups recovered with their dams for 2 h. The groups were treated with WEB 2170 10 mg/kg s.c. or saline solution 1 h before exposure to hypoxia. At 14 d of recovery from HI, the rats were killed, and the percentage right hemisphere weight deficit was calculated.
Experiment 4: Evaluation of brain atrophy with WEB 2170 pre- andposttreatment:.
To determine whether long-term neuroprotection required pre- and posttreatment with WEB 2170, we performed an additional experiment in which rat pups were randomized after carotid ligation to receive saline solution or repeated doses of WEB 2170. The PAF antagonist was given at 1 h before, and again at 0 h and 48 h after HI. At 14 d of recovery from HI, the rats were killed, and the percentage right hemisphere weight deficit was calculated.
Experiment 5: Evaluation of atrophy with WEB 2170 and BN52021.
To determine whether long-term neuroprotection could be achieved with either WEB 2170 or BN 52021 (a PAF antagonist that readily crosses the blood-brain barrier), we performed an additional experiment in which rat pups were randomized after carotid ligation into three groups. The first group was treated with WEB 2170 (10 mg/kg s.c.) 1 h before hypoxia, the next group received BN 52021 (25 mg/kg) 5 min before and again 1 h after hypoxia, and the control group received saline solution administered 1 h before hypoxia. BN 52021 was obtained from Biomol Inc (Plymouth Meeting, PA, U.S.A.) and was suspended in 5% gum arabic (Sigma Chemical Co., St. Louis, MO, U.S.A.) in water. The WEB 2170 dose we used was chosen because we had found it reduced brain swelling. The BN 52021 dosing schedule and method of administration was intended to mimic the neuroprotective studies performed by Liu et al. (14).
Statistical analyses
To determine the statistical significance of the difference between treatment groups we used nonparametric tests: Mann-Whitney U test for two groups and Kruskal-Wallis test for more than two groups. A p < 0.05 was considered to be significant. All values are given as mean ± SD.
RESULTS
The results are summarized in Table 1.
Brain swelling with WEB 2170 before HI insult (experiment 1).
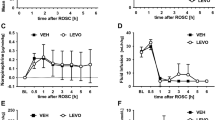
Right hemisphere swelling was reduced by 64% in the WEB 2170-treated rats. The right hemisphere swelled 13.6 ± 9.8% in the 22 saline-treated rats whereas in the 20 WEB 2170-treated rats, it swelled only 5.0 ± 8.9% (p < 0.01, Mann-Whitney U test). One WEB 2170-treated pup died during hypoxia. No saline-treated rats died during hypoxia. No pups died during the 42-h recovery period. The WEB2170-treated pups gained 40% more weight than the saline-treated pups (p < 0.01, Mann-Whitney U test). The 22 saline-treated pups gained 13.8 ± 7.7% weight in the 42-h recovery interval whereas the WEB 2170-treated rats grew 18.6 ± 1.5% (p = 0.03).
Brain swelling with WEB 2170 after HI insult (experiment 2).
In contrast to the reduction in brain swelling produced by pretreatment, treatment immediately after HI injury was not protective. Right hemisphere swelling was not reduced by either the 1- or the 10-mg/kg dose. The right hemispheres swelled 20.0 ± 7.3%, 18.4 ± 9%, and 19.3 ± 8.5% in the saline-, 1-mg/kg WEB 2170-, and 10-mg/kg WEB 2170-treated rat pups, respectively (n = 15 each group;p = 0.958, Kruskal-Wallis test).
No pups died during the 42-h recovery period. There was no significant difference in weight gain between groups.
Brain atrophy with WEB 2170 before HI insult (experiment 3).
Brain atrophy was not reduced with pretreatment. The percentage interhemispheric weight deficit measured was 21.2 ± 16.2% in the 27 saline-treated pups and 20.9 ± 16.7% in the 32 WEB 2170-treated pups (p = 0.99, Mann-Whitney U test). No pups died during the 14-d recovery period in either group.
Brain atrophy with WEB 2170 before and after HI insult (experiment4).
Repeated treatment with WEB 2170 before and after HI insult did not prevent brain atrophy. Atrophy was 67.0 ± 20.8% and 66.6 ± 23.8% in the 26 saline-treated rats and the 28 WEB 2170-treated rats, respectively (p = 0.83, Mann-Whitney U test). No pups died during the long-term recovery period in either group.
Brain atrophy with WEB 2170 before HI insult or BN 52021 before andafter HI insult (experiment 5).
Neither PAF antagonist reduced brain atrophy. Atrophy was 23.5 ± 21.3% in the 27 saline-treated rats; 14.7 ± 18.1% in the 26 WEB 2170-treated rats (p = 0.11 versus saline); and 21.2 ± 21.5% in the 24 BN 52021-treated rats (p = 0.7 versus saline, ANOVA). We determined that there was 80% power to detect a difference of >16% weight deficit in either treatment group versus the saline-treated group.
DISCUSSION
This study found that post-HI brain swelling in P7 rats could be reduced by pretreatment with the PAF antagonist WEB 2170. Despite the ability of pretreatment to reduce brain swelling, WEB 2170 did not reduce cerebral atrophy or infarction. Treatment immediately after the HI insult with WEB 2170 was not protective in regards to atrophy or swelling. This study also found that brain atrophy could be not be reduced with another PAF antagonist, BN 2021.
Brain swelling from vasogenic edema increases progressively for 72 h in the neonatal rat (21). Imaging studies of the neonatal rat brain after hypoxia-ischemia as measured by sequential repeated transversal relaxation time (T2) -weighted images confirmed that vasogenic edema increases progressively during the first 72 h of recovery (22). PAF receptor antagonists decrease vascular injury and permeability (23). Therefore, the reduction in brain water content and swelling we found with WEB 2170 pretreatment probably represents a reduction in vasogenic edema.
WEB 2170 was unable to reduce brain swelling when it was administered immediately on recovery from hypoxia-ischemia. This indicates that PAF injured blood vessels during the insult or very early during HI recovery. In support of the notion that PAF was expressed early and transiently, Domingo et al. (24) found that in adult gerbils, there was an almost 10-fold increase in PAF immediately after 10 min of cerebral ischemia. PAF concentrations returned to normal after 30 min of reperfusion. Although PAF expression is transient, it acts via receptors and secondary messengers, which prolong its action. PAF has been shown to increase intracellular calcium (25) and enhance production of free radicals (26). In excess, PAF can injure neurons by inhibiting Na+/K+ ATPase activity and by disrupting energy metabolism (11).
In untreated P7 rats subjected to the HI insult, the severity of brain swelling correlates with the extent of tissue damage (2). In this study, we administered a PAF antagonist that was confined to the intravascular compartment and observed reduced edema but not atrophy. This indicates that the pathogenesis of parenchymal injury does not depend to a large extent on the degree of brain swelling in the neonatal rat. This interesting observation has not to our knowledge been previously reported, although we have described the same phenomenon in preliminary studies with other neuroprotective agents that do not readily cross the blood-brain barrier (27, 28).
In adult animals, the skull bones are fused, and brain swelling may impair cerebral perfusion pressure. Accordingly, in adult animal models of ischemic brain injury, it remains possible that drugs that reduce damage to blood vessels may prevent secondary ischemia from brain swelling (even if they do not cross the blood-brain barrier). Hence, in mature animals, it remains possible for pharmacologic agents that do not cross the blood-brain barrier to reduce brain injury.
The blood-brain barrier is a lipid membrane, and its penetrability is inversely related to the tendency of a compound to dissolve in lipid (29). WEB 2170 has a hetrazepine structure with predominant hydrophilic properties. It is poorly soluble in lipid as indicated by its low partition coefficient (30). In contrast to WEB 2170, BN 52021 is a lipid-soluble PAF antagonist that reduced cerebral infarction by about 60% in a similar P7 rat model of HI brain injury (14). We initially thought that WEB 2170 failed to reduce atrophy because it did not cross the blood-brain barrier. To investigate this hypothesis, we compared WEB 2170 with BN 52021in their ability to reduce atrophy after cerebral hypoxia-ischemia. Interestingly, we found that neither PAF antagonist reduced atrophy. As we were able to reduce swelling by 64% with the same dose and administration of WEB 2170, we believe that the lack of effect on atrophy is important. The study was statistically powered to detect with 80% certainty a difference of 16% in brain atrophy. Thus, smaller differences (neuroprotective effects) could have gone undetected.
It is not clear why our results differed from those of Liu et al. (14), as the rat pups in both studies received similar doses of BN 52021. One possible factor might be slight differences in experimental conditions, such as ambient temperature, or rat strains. We used Wistar rats whereas the other investigators used Sprague-Dawley rats (14). Strain differences in experimentally induced infarct volume have been reported: Duverger and MacKenzie (31) compared infarct volume after occlusion of the middle cerebral artery in five strains of adult rats and found that Wistar rats had smaller infarcts than Sprague-Dawley rats.
In summary, WEB 2170 is a PAF antagonist that acts within the microvascular compartment to reduce post-HI brain swelling. The need to administer it before hypoxia-ischemia suggests that PAF is an early mediator of vascular injury and that PAF injures blood vessels during the insult or very early during reperfusion. This study also suggests that post-HI brain swelling does not contribute significantly to injury because injury is unaffected when brain swelling is reduced. In addition, as we were unable to reduce brain atrophy with either WEB 2170 or BN 52021, it suggests that in the neonatal Wistar rat, PAF does not contribute significantly to parenchymal injury.
Abbreviations
- PAF:
-
platelet activating factor
- P7:
-
7-day old rats
- HI:
-
hypoxic-ischemic
References
Palmer C 1995 Hypoxic-ischemic encephalopathy: therapeutic approaches against microvascular injury, and role of neutrophils, PAF, and free radicals. Clin Perinatol 22: 481–517
Vannucci RC, Christensen MA, Yager JY 1993 Nature, time-course, and extent of cerebral edema in perinatal hypoxic-ischemic brain damage. Pediatr Neurol 9: 29–34
Satoh K, Yoshida H, Imaizumi TA, Koyama M, Takamatsu S 1995 Production of platelet-activating factor by porcine brain microvascular endothelial cells in culture. Thromb Haemost 74: 1335–1339
Bratton D, Henson PM 1989 Cellular origin of PAF. In: Barnes PJ, Page CP, Henson PM (eds) Platelet Activating Factor in Human Disease. Blackwell Scientific, London, pp 23–57
Humphrey DMN, McManus LM, Hanahan DJ, Pinckard RN 1984 Morphologic basis of increased vascular permeability induced by acetyl glyceryl ether phosphorylcholine. Lab Invest 50: 16–25
Maki N, Hoffman DR, Johnston JM 1988 Platelet activating factor acetylhydrolase activity in maternal, fetal and newborn rabbit plasma during pregnancy and lactation. Proc Natl Acad Sci U S A 85: 728–732
Francescangeli E, Goracci G 1989 The de novo biosynthesis of platelet-activating factor in rat brain. Biochem Biophys Res Commun 161: 107–112
Sogos V, Bussolino F, Pilia E, Torelli S, Gremo F 1990 Acetylcholine-induced production of platelet-activating factor by human fetal brain cells in culture. J Neurosci Res 27: 706–711
Predescu D, Ihida K, Predescu S, Palade GE 1996 The vascular distribution of the platelet-activating factor receptor. Eur J Cell Biol 69: 86–98
Bito H, Honda Z, Nakamura M, Shimizu T 1994 Cloning, expression and tissue distribution of rat platelet-activating factor receptor cDNA. Eur J Biochem 221: 211–218
Spinnewyn B, Blavet N, Clostre F, Bazan N, Braquet P 1987 Involvement of platelet-activating factor (PAF) in cerebral post-ischemic phase in mongolian gerbils. Prostaglandins 34: 337–349
Lindsberg PJ, Hallenbeck JM, Feuerstein G 1991 Platelet-activating factor in stroke and brain injury. Ann Neurol 30: 117–129
Yue TL, Gleason MM, Gu JL, Lysko PG, Hallenbeck J, Feuerstein G 1991 Platelet-activating factor (PAF) receptor-mediated calcium mobilization and phosphoinositide turnover in neurohybrid NG108–15 cells: studies with BN 50739, a new PAF antagonist. J Pharmacol Exp Ther 257: 374–381
Liu XH, Eun BL, Silverstein FS, Barks JDE 1996 The platelet-activating factor antagonist BN 52021 attenuates hypoxic-ischemic brain injury in the immature rat. Pediatr Res 40: 797–803
Faden AI, Tzendzalian PA 1992 Platelet-activating factor antagonists limit glycine changes and behavioral deficits after brain trauma. Am J Physiol 263: R909–R914
Rice JE, Vannucci RC, Brierley JB 1981 The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 9: 131–141
Palmer C, Towfighi J, Roberts RL, Heitjan DF 1993 Allopurinol administered after inducing hypoxia-ischemia reduces brain injury in 7-day-old rats. Pediatr Res 33: 405–411
Gidday JM, Fitzgibbons JC, Shah AR, Park TS 1994 Neuroprotection from ischemic brain injury by hypoxic preconditioning in the neonatal rat. Neurosci Lett 168: 221–224
Anderson BO, Poggetti RS, Shanley PF, Bensard DD, Pitman JM, Nelson DW, Whitman GJ, Banerjee A, Harken AH 1991 Primed neutrophils injure rat lung through a platelet-activating factor dependent mechanism. J Surg Res 50: 510–514
Zhou W, McCollum MO, Levine BA, Olson MS 1992 Inflammation and platelet-activating factor production during hepatic ischemia/reperfusion. Hepatology 16: 1236–1240
Vannucci RC, Christensen MA, Yager JY 1993 Nature, time-course and extent of cerebral edema in perinatal hypoxic-ischemic brain damage. Pediatr Neurol 9: 29–34
Rumpel H, Nedelcu J, Aguzzi A, Martin E 1997 Late glial swelling after acute cerebral hypoxia ischemia in the neonatal rat: a combined magnetic resonance and histochemical study. Pediatr Res 42: 54–59
Lehr HA, Seemuller J, Hubner C, Menger MD, Messmer K 1993 Oxidized LDL-induced leukocyte/endothelium interaction in vivo involves the receptor for platelet-activating factor. Arterioscler Thromb 13: 1013–1018
Domingo MT, Spinnewyn B, Chabrier PE, Braquet P 1994 Changes in [3H]PAF binding and PAF concentrations in gerbil brain after bilateral common carotid artery occlusion: a quantitative autoradiographic study. Brain Res 640: 268–276
Kornecki E, Ehrlich YH 1988 Neuroregulatory and neuropathological actions of the ether-phospholipid platelet-activating factor. Science 240: 1792–1794
Matsuo Y, Kihara T, Ikeda M, Ninomiya M, Onodera H, Kogure K 1996 Role of platelet-activating factor and thromboxane A2 in radical production during ischemia and reperfusion of the rat brain. Brain Res 709: 296–302
Palmer C, Roberts RL 1997 Deferoxamine starch conjugate provides partial protection from hypoxic-ischemic brain injury in neonatal rats. Pediatr Res 41: 294Aabstr
Palmer C, Roberts RL, Young P 1996 The reduction of hypoxic-ischemic brain injury in the 7-day old rat with PEG-SOD. Pediatr Res 39: 379Aabstr
Bradbury M 1979 The barrier to drugs, neurotransmitters and metabolites. In: Bradbury M (ed) The Concept of a Blood-Brain Barrier. John Wiley & Sons, Chichester, pp 323–350
Weber KH, Heuer H 1989 Structure-activity relationships and effects of platelet-activating factor antagonists in the hetrazepine series. Int Arch Allergy Appl Immunol 88: 82–87
Duverger D, MacKenzie ET 1988 The quantification of cerebral infarction following focal ischemia in the rat: influence of strain, arterial pressure, blood glucose concentration, and age. J Cereb Blood Flow Metab 8: 449–461
Acknowledgements
The authors thank Michael S. Caplan, M.D., Department of Pediatrics, Evanston Hospital, North Western University Medical School, Chicago, IL, U.S.A., for his assistance in this study.
Author information
Authors and Affiliations
Additional information
Supported by National Institutes of Health grants NS29704 and HD31704 to C.P.
Rights and permissions
About this article
Cite this article
Viswanath, M., Palmer, C. & Roberts, R. Reduction of Hypoxic-Ischemic Brain Swelling in the Neonatal Rat with PAF Antagonist WEB 2170: Lack of Long-Term Protection. Pediatr Res 48, 109–113 (2000). https://doi.org/10.1203/00006450-200007000-00019
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200007000-00019
This article is cited by
-
Loss of PAFR prevents neuroinflammation and brain dysfunction after traumatic brain injury
Scientific Reports (2017)