Abstract
Background
Systemic inflammation amplifies neonatal hypoxic–ischemic (HI) brain injury. Azithromycin (AZ), an antibiotic with anti-inflammatory properties, improves sensorimotor function and reduces tissue damage after neonatal rat HI brain injury. The objective of this study was to determine if AZ is neuroprotective in two neonatal rat models of inflammation-amplified HI brain injury.
Design/Methods
Seven-day-old (P7) rats received injections of toll-like receptor agonists lipopolysaccharide (LPS) or Pam3Cys-Ser-(Lys)4 (PAM) prior to right carotid ligation followed by 50 min (LPS + HI) or 60 min (PAM + HI) in 8% oxygen. Outcomes included contralateral forelimb function (forepaw placing; grip strength), survival, %Intact right hemisphere (brain damage), and a composite score incorporating these measures. We compared postnatal day 35 outcomes in controls and groups treated with three or five AZ doses. Then, we compared P21 outcomes when the first (of five) AZ doses were administered 1, 2, or 4 h after HI.
Results
In both LPS + HI and PAM + HI models, AZ improved sensorimotor function, survival, brain tissue preservation, and composite scores. Benefits increased with five- vs. three-dose AZ and declined with longer initiation delay.
Conclusions
Perinatal systemic infection is a common comorbidity of neonatal asphyxia brain injury and contributes to adverse outcomes. These data support further evaluation of AZ as a candidate treatment for neonatal neuroprotection.
Impact
-
AZ treatment decreases sensorimotor impairment and severity of brain injury, and improves survival, after inflammation-amplified HI brain injury, and this can be achieved even with a 2 h delay in initiation.
-
This neuroprotective benefit is seen in models of inflammation priming by both Gram-negative and Gram-positive infections.
-
This extends our previous findings that AZ treatment is neuroprotective after HI brain injury in neonatal rats.
Similar content being viewed by others
Introduction
Neonatal encephalopathy (NE) affects 1–4/1000 live term or near-term births in developed countries.1 Moreover, in low-middle income countries NE is up to 10-fold more frequent2 and it is a leading cause of disability and early death.3 Although NE has often presumptively been attributed to antecedent fetal ischemia and/or hypoxia, there is increasing recognition of multiple potential contributing mechanisms and etiologies.4,5 Maternal or fetoplacental infections are common predisposing factors or comorbidities of NE,6 and are associated with heightened risks for adverse long-term neurodevelopmental outcomes.7 Yet, when infants present acutely with NE in the setting of maternal chorioamnionitis, the responsible pathogen is usually unknown and most often the neonate does not have positive blood or cerebrospinal fluid cultures.8 Among the many intraamniotic organisms associated with chorioamnionitis in term infants,9,10 the Gram-positive organism Group B Streptococcus is the most common invasive pathogen identified, and the Gram-negative Escherichia coli is the second most common.11,12 Thus, evaluation of interactions between both Gram-positive and Gram-negative organisms and acute hypoxic–ischemic (HI) injury in relevant neonatal models is important.
The complex pathophysiologic interactions between perinatal infection and acute HI brain injury can be modeled experimentally.13 The best-defined strategies incorporate systemic administration of a pro-inflammatory stimulus, most commonly a toll-like receptor (TLR) agonist, followed by a standard lesioning method to elicit unilateral HI brain injury. Hagberg and colleagues first showed that systemic injection of a very low dose of E. coli-derived lipopolysaccharide (LPS, a TLR4 agonist) heightened vulnerability to HI brain injury, elicited by unilateral carotid artery ligation and timed exposure to moderate hypoxia in immature (7-day-old) rats.14 In the neonatal HI lesion model upon which this study was based, the severity of brain injury is dependent on the duration of hypoxia exposure;15,16 with antecedent LPS administration, the temporal thresholds for brain injury were substantially reduced,14 i.e., shorter durations of hypoxia resulted in brain injury. These findings suggested that systemic infections with Gram-negative bacteria could heighten susceptibility to neonatal HI brain injury. LPS-induced post-HI hypoglycemia was suggested as the underlying mechanism, but treatment with supplemental glucose only partially attenuated LPS-amplified brain injury.17 Subsequent work implicated LPS-activated microglia as mediators of amplified brain injury in the rodent model.18,19,20
Neonates are susceptible to both Gram-negative and Gram-positive bacterial infections. Bacteria-derived lipoproteins that activate TLR2 represent a pivotal pathogenic mechanism in group B streptococcal sepsis.21 Interest in understanding whether Gram-positive bacteria-mediated TLR2 activation could exert similar deleterious effects to the E. coli-derived TLR4 agonist prompted complementary studies with a synthetic lipopeptide TLR2 agonist Pam3Cys-Ser-(Lys)4 (Pam3CSK4, “PAM”).22,23,24 In fact, PAM pretreatment also amplified HI brain injury, but distinct differences between the two pro-inflammatory stimuli emerged, with respect to both physiological adverse effects (more pronounced with LPS) and magnitude and temporal profile of brain cytokine expression.22
Azithromycin (AZ), a generally safe, widely used antibiotic, has intrinsic anti-inflammatory properties,25 and accumulates in brain tissue.26,27 Evidence of AZ’s safety in human neonates was provided by the results of a series of studies that were undertaken to evaluate the impact of AZ treatment on Ureaplasma eradication in premature infants <29 weeks gestation at birth.28,29,30,31 In view of the pivotal role of inflammation in the pathogenesis of diverse forms of neonatal brain injury,13 we initially evaluated AZ’s neuroprotective efficacy in the neonatal rat HI brain injury model, elicited by unilateral carotid artery ligation and subsequent hypoxia exposure; AZ treatment, even when initiated several hours after the end of hypoxia, improved functional outcomes and reduced brain injury.32 Congruent findings are reported in adult rodent stroke.33
In this study, building on our findings and knowledge of AZ’s immune-modulatory properties, we hypothesized that AZ could attenuate inflammation-amplified HI injury and incorporated experiments to evaluate AZ neuroprotection in both LPS- and PAM-amplified HI brain injury models. We selected AZ treatment protocols that incorporated delayed onset of initial drug administration (from 1 to 4 h after the end of hypoxia exposure) and multiple-dose regimens (up to five doses) to model translationally feasible clinical scenarios.
Methods
Overview
Our general approaches include randomizing concurrently lesioned animals from a single litter to drug-treated and saline control groups, and distributing males and females evenly among groups. We only include non-lesioned littermate controls when we are evaluating a functional outcome that is non-lateralizing, such as a “cognitive” measure. Dose selection and timing of AZ administration were guided by the results of prior studies in our laboratory.32,34 In pilot experiments, based on our experiences and protocols from other laboratories,35,36,37 we identified LPS and PAM administration protocols (dose and timing), coupled with hypoxia–ischemia durations, that yielded brain injury and relatively low mortality.
All outcome assessments included comparisons of performance on two sensorimotor function measures (see below) and quantification of brain injury. We implemented a novel approach to integrate function and pathology measures, and mortality, by calculating composite outcome scores (see below). In initial neuroprotection experiments, sensorimotor testing was continued weekly until postnatal day 35 (P35) and brain injury was evaluated in coronal histopathology sections. In subsequent experiments to evaluate the impact of timing of treatment onset on neuroprotective efficacy, outcomes were evaluated on P21. All protocols were approved by the University of Michigan Animal Care and Use Committee.
Animal lesioning
Isofluorane-anesthetized P7 Wistar rats underwent right carotid artery ligation, recovered in incubators (90 min, at 36.5 °C, Hovabator, GQF, Savannah, GA), were placed in covered acrylic containers partially submerged in a water bath (36.5 °C), and exposed to 8% oxygen/balance nitrogen,32 for a hypoxia duration as specified for each protocol below. They recovered in incubators (15 min, 36.5 °C), and then returned to dams.
Pro-inflammatory challenges
To model the clinical scenario of HI brain injury in the setting of Gram-negative systemic infection, LPS (E. coli, O55:B5, Sigma-Aldrich, St. Louis, MO, 0.05 mg/kg), a TLR4 agonist, was injected intraperitoneally (i.p.), 2.5 h prior to carotid ligation, and 1.5 h post ligation, exposure to 50 min 8% O2 began;20,35 this protocol is designated as “LPS + HI.” To model concurrent Gram-positive systemic infection, the synthetic lipopeptide TLR2 agonist Pam3Cys-Ser-(Lys)4 (Pam3CSK4, PAM, Sigma-Aldrich, 0.5 mg/kg) was injected i.p. 4.5 h prior to carotid ligation, and 1.5 h post ligation, exposure to 60 min 8% O2 started;37 this protocol is designated as “PAM + HI.”
Physiology measurements
Animals were weighed on P7, on each subsequently scheduled injection day, and then weekly from P14. Rectal temperatures were uniformly measured (YSI thermometer 43T with probe 554; YSI, Yellow Springs, OH) before surgery, at the end of hypoxia, and 15 min, 60 min, 120 min, and 24 h later, and also at 48, 72, and 96 h in one LPS + HI experiment and in all PAM + HI experiments. Blood glucose was measured in representative litters at several time points after HI, including immediately before AZ, and up to 24 h after HI (Germaine Laboratories AimStrip Plus Blood Glucose Testing System and Test Strips, Germaine Laboratories, San Antonio, TX).
AZ treatment
Sterile lyophilized AZ (Fresenius Kabi USA, Lake Zurich, IL) was dissolved in sterile 0.9% saline, as required to achieve uniform i.p. injection volumes of 0.1 ml per 10 g body weight; routine intravenous injections are not feasible at this age. Controls were injected with 0.1 ml per 10 g body weight of sterile saline. In both LPS + HI and PAM + HI models, we compared P35 outcomes of three-dose (initial 45 mg/kg at 2 h after HI, followed by 22.5 mg/kg at 24 and 48 h after HI, “AZ*3”) and five-dose AZ regimens (adding 22.5 mg/kg doses at 72 and 96 h after HI, “AZ*5”) with outcomes in saline-injected controls (see Table 1). In subsequent experiments in each model, we evaluated the impact of the timing of treatment onset on the efficacy of the five-dose AZ regimen, comparing initial-dose administration at 1, 2, or 4 h after the end of HI with saline-injected controls; outcomes were assessed at P21 (see Table 1).
Sensorimotor function testing
Performance was evaluated with two independent tests that consistently demonstrate asymmetric sensorimotor deficits in this model; videos of these tests are available online.32 Vibrissae-stimulated forepaw placing (10 trials/side) was tested weekly from P14 up to P21 or P35; one point was assigned for full and 0.5 points for partial extension (normal = 10/10 bilaterally).38,39 Right forepaw placement was consistently normal, and only left forepaw scores are reported. Forepaw grip strength was measured (3 trials/side, Bioseb Grip Traction Meter model BIO-GS3, Forceleader DBA Bioseb, Pinellas Park, FL) on P21 (youngest age at which reliable measures can be obtained) or weekly from P21 to P35.39 In normal rats, grip strength increases from P21 to P35 and remains bilaterally symmetric. To account for expected age-related increases in grip strength, results are expressed as left/right (L/R) forepaw grip strength ratios. After P7 HI lesioning, performance deficits on both measures are similar at P21 and P3532 (also see Supplementary Data Table S4).
Brain injury measures
Animals were euthanized on P35 or P21 by i.p. pentobarbital injection (Fatal Plus®, Vortech Pharmaceuticals, Dearborn, MI). In experiments that evaluated P35 outcomes, brains were rapidly frozen, and coronal brain sections (20 μm) were cresyl violet stained. Bilateral cross-sectional areas of the striatum, neocortex, hippocampus, and entire cerebral hemisphere were measured (using NIH Image J) on regularly spaced sections (mean 19 sections/brain; distance between sections 256 μm in LPS + HI experiments and 320 μm in PAM + HI experiments) from the level of the anterior genu to the posterior genu of the corpus callosum. Bilateral regional and hemisphere intact tissue volumes were calculated by summing areas and multiplying the sum by the distance between sections.39 As measures of injury severity, right-sided %Intact regional or whole hemisphere tissue volumes were expressed as [100 × (right volume)/(left volume)]. In experiments that compared outcomes at P21, right and left cerebral hemispheres were separated and weighed; the difference in hemisphere weight is a validated measure of hemisphere volume difference in this model.40
For composite outcome score calculations (see below), either %Intact right hemisphere volume (P35) or % right hemisphere weight relative to left [100 × (right/left hemisphere weights)] values (P21) were included.
Composite outcome score
To generate a global outcome measure that would also take into account survival differences, we developed a 30-point composite outcome incorporating the two function measures, %Intact right hemisphere and survival. The best possible composite score is 30. Scores were calculated as: {[left forepaw vibrissae score (maximum = 10)] + [(L/R forepaw grip ratio) × 10] + [(%Intact right hemisphere)/10]}. Animals that died before the completion of testing were assigned scores of 0 for each measure, and these values were included in all outcome assessments.
Data analysis
Sequential rectal temperatures and body weights were compared by repeated-measures analysis of variance (ANOVA) (using Prism 8.4.3, GraphPad Software, San Diego, CA, for all analyses). Impact of AZ treatment on blood glucose values after HI was evaluated by t test or repeated-measures ANOVA. Nonparametric Kruskal–Wallis testing was applied to compare sensorimotor measures, %Intact right hemisphere and composite score among AZ treatment groups and controls, since, by visual inspection, data were not normally distributed in many groups. To evaluate the decrement in AZ benefit as treatment delay increased, a post hoc ANOVA linear test for trend was performed across ordered AZ 1 h, 2 h, 4 h, and normal saline control (NS) groups. Two-way ANOVA, factoring group and age was applied to compare sensorimotor test scores at P21 and P35 in both models, and also, factoring treatment and sex, to evaluate for sex differences in the impact of AZ treatment on sensorimotor testing measures, %Intact right hemisphere, and composite score. Repeated-measures ANOVA, factoring region (as a repeated measure), and treatment was applied to evaluate regional specificity of AZ treatment effects.
Results
Physiology
In studies of P7 rat HI lesioning that incorporate hypoxia exposure durations of at least 90 min, typical mortality rates are <5%. Based on the anticipated deleterious effects of LPS and PAM, in these “double-hit” models, hypoxia durations were shortened. Yet, in both models post-HI mortality was relatively high in saline-treated controls: LPS + HI (5/27; 19%) and PAM + HI (3/13; 23%). In the LPS + HI model, mortality was significantly reduced in AZ-treated groups (saline 5/27 vs. AZ 3/67; p < 0.05 Fisher’s exact test). In the PAM + HI model, there was a nonsignificant trend to lower mortality in the AZ groups (saline 3/13 vs. AZ 1/32; p = 0.07, Fisher’s exact test).
AZ treatment had no impact on body temperature for up to 4 days after HI (Supplementary Data Table S1, available online). Body weights from P8 through P21 or P35 did not differ between AZ-treated and control groups (not shown). Post-HI blood glucose concentrations differed between models; LPS-exposed animals were hypoglycemic, whereas values in PAM-exposed animals were in the same range as controls. AZ treatment did not alter blood glucose concentrations in either group, and values in all groups were in the normal range at 24 h post HI (see Supplementary Data Table S2).
AZ improves outcomes of LPS-amplified HI injury
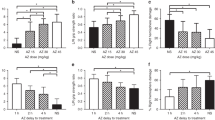
Figure 1, panels a–d, compares P35 function and brain injury measures, along with composite score in three LPS + HI groups: saline controls (NS), animals that received three doses of AZ (AZ*3), and animals that received five AZ doses (AZ*5). Left forepaw placement (scored in 10 trials, Fig. 1a) was markedly impaired in NS controls, significantly better in the AZ*3 group, and close to normal in the AZ*5 group (p < 0.0001 Kruskal–Wallis; p < 0.05 Dunn’s multiple comparisons test for all three between-group differences). Left(L)/right (R) grip strength ratios (normal = 1) were markedly reduced in NS controls, significantly higher in the AZ*5-treated group, and higher in the AZ*5 than AZ*3 group (p < 0.0001 Kruskal–Wallis; p < 0.05 Dunn’s multiple comparisons test for NS vs. AZ*5 and AZ*3 vs. AZ*5). The extent of right cerebral hemisphere volume loss (Fig. 1c) was more variable and significantly different from controls only in the AZ*5 group (p < 0.005 Kruskal–Wallis with Dunn’s multiple comparisons test, also see Table 2). Overall, composite scores (best possible = 30) were significantly higher than controls in both AZ*3 and AZ*5 groups (p < 0.0001, Kruskal–Wallis), and better in AZ*5 than AZ*3 groups (p < 0.05 Dunn’s).
P7 rats received i.p. LPS injections 4 h prior to initiation of 50 min HI (LPS + HI), and were allocated to three-dose AZ (AZ*3), five-dose AZ (AZ*5), or saline control (NS) groups (see “Methods”). Panels a–c compare sensorimotor function and % residual intact right hemisphere volumes on P35; panel d compares outcomes with a 30-point composite score that incorporates these measures and also reflects survival (see “Methods”). Horizontal bars represent median and IQR. Both AZ treatments improved composite score vs. NS (d: p < 0.0001 Kruskal–Wallis test). Composite score and functional performance were superior in AZ*5 vs. AZ*3 groups (a, b, d; *p < 0.05 Dunn’s multiple comparisons test); all AZ*5 scores were superior to controls, as were AZ*3 sensorimotor test scores.
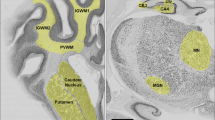
Figure 2, panels a–f, illustrates the features of P35 histopathology at the levels of the striatum (a, c, e) and dorsal hippocampus (b, d, f) in samples from each of the three LPS + HI groups: saline controls (a, b), the three-dose AZ regimen (c, d; AZ*3), or the five-dose AZ regimen (e, f; AZ*5). In the LPS + HI control (a, b), overall right hemisphere, striatal, and hippocampal atrophy are evident, as well as cortical thinning and cystic encephalomalacia. The AZ*3 sample (c, d) demonstrates greater tissue preservation, with mild right hemisphere and striatal atrophy, and minimal hippocampal atrophy. In the AZ*5 sample (e, f), only very mild right striatal (*) and hippocampal tissue loss are evident. Table 2a provides results of regional morphometry to evaluate the extent and distribution of tissue injury in each treatment group. % Intact values, calculated as 100 × (R/L) volumes were included as indices of tissue damage. Although there was variability in the extent of tissue damage within each group, as commonly encountered in the P7 rat HI model, these data confirmed that AZ*5 conferred significant neuroprotection across all regions while AZ*3 benefits were more variable.
Panels a–f illustrate representative cresyl violet-stained coronal brain sections, obtained at P35, in samples from the three groups compared in Fig. 1. a–d P7 rats received LPS injections followed by 50 min HI (LPS + HI; see “Methods”). Two hours after the end of HI, animals started treatment with saline (a, b), the three-dose AZ regimen (c, d; AZ*3), or the five-dose AZ regimen (e, f; AZ*5). Sections a, c, and e are at the level of the striatum (*); sections b, d, and f are at the level of the dorsal hippocampus (arrows). In the LPS + HI control (a, b), note overall right hemisphere atrophy, striatal atrophy (*), marked hippocampal atrophy (arrow), and cortical thinning and cystic encephalomalacia (arrowheads). The AZ*3 sample (c, d) demonstrates mild right hemisphere and striatal atrophy (*), and minimal hippocampal atrophy (arrow). In a representative AZ*5 animal (e, f), only very mild right striatal (*) and hippocampal (arrow) tissue loss are evident. Scale bar = 2 mm.
In the preceding experiments, a treatment onset time point 2 h after the end of hypoxia was selected, based both on the goal of modeling a translationally relevant clinical scenario and also on the knowledge that AZ treatment, initiated at this time point, was neuroprotective in P7 rats that underwent 90 min HI.32 To re-evaluate the impact of timing of treatment onset in the LPS + HI model, we focused on the AZ*5 regimen and compared outcomes on P21 when the first AZ dose had been administered 1, 2, or 4 h after the end of HI. Figure 3, panels a–d, summarizes the results. Median composite scores at P21 (and each component measure) were superior to control values when AZ was initiated at 1 or 2 h, but not at 4 h after the end of HI (Fig. 3a–d, p < 0.0001, Kruskal–Wallis; *p ≤ 0.0001 Dunn’s post hoc). A linear decrement in AZ efficacy with increasing delay in treatment initiation was evident (p < 0.0001 post hoc linear test for trend and a trend toward some benefit vs. controls even in the 4 h group).
Outcomes of AZ*5 regimens with initial-dose administration at 1, 2, or 4 h after the end of HI were compared with NS controls (a–d) were assessed at P21; %Intact right hemisphere (c) was calculated using bilateral hemisphere weights. AZ*5 treatment initiated at 1 or 2 h post HI, but not at 4 h, conferred improvements in each measure and composite score compared to controls (p < 0.0001, Kruskal–Wallis, *p ≤ 0.0001 Dunn’s). A linear decrement in AZ efficacy with increasing delay in treatment initiation was evident (p < 0.0001 post hoc linear test for trend).
We incorporated both P35 and P21 composite scores to evaluate if there were sex differences in AZ efficacy (Supplementary Data Table S3). Composite scores did not differ in saline controls (mean ± SD: female: 10.1 ± 6.0, male: 10.1 ± 5.1) or in AZ*5-treated groups (female: 23.3 ± 7.1; male: 22.4 ± 7.2).
AZ improves outcomes of PAM-amplified HI injury
Note that based on prior studies and our pilot data (not shown), we implemented a PAM + HI model that differed from the LPS + HI model both with respect to the time interval between PAM administration and subsequent HI lesioning (6 h, rather than 4 h earlier), and HI duration (60 min rather than 50 min). Overall, AZ neuroprotective efficacy trends were similar in both models. Figure 4, panels a–d, compares P35 function and brain injury measures, along with composite scores in three corresponding PAM + HI groups: saline controls (NS), animals that received three doses of AZ (AZ*3), and animals that received five AZ doses (AZ*5). Controls all had significant sensorimotor deficits (Fig. 4a, b), and right hemisphere brain damage (0 values represent animals that died prior to P35). Both AZ*5 and AZ*3 improved scores on all measures, compared with controls (p < 0.005 Kruskal–Wallis); however, there were no significant differences in outcomes between AZ*5 and AZ*3 groups in these analyses. Regional morphometry measures (Table 2b) demonstrated similar trends, although significantly greater tissue preservation for both AZ treatments was only found in comparisons of striatal and hemisphere volumes (p < 0.05, ANOVA and post hoc Tukey’s tests).
P7 rats received i.p. injections of Pam3CSK4 6 h prior to 60 min HI (PAM + HI), and were allocated to three-dose AZ (AZ*3), five-dose AZ (AZ*5), or saline control (NS) groups (see “Methods”). Panels a–c compare sensorimotor function and %Intact right hemisphere volumes on P35 in the three groups; panel d compares outcomes with a 30-point composite score that incorporates these measures and also reflects survival (see “Methods”). Horizontal bars represent median and IQR. There was a significant AZ treatment benefit by all measures (p < 0.005 Kruskal–Wallis). In both AZ treatment groups, for each measure results were superior to values in controls (*p < 0.05, Dunn’s multiple comparisons test); scores did not differ significantly between the AZ*5 and AZ*3 groups.
Figure 5 illustrates histopathology in samples from each of the groups compared in Fig. 4a–d at the levels of the striatum (a, c, e) and dorsal hippocampus (b, d, f) in samples from each of the three PAM + HI groups: saline controls (a, b), the three-dose AZ regimen (c, d; AZ*3), or the five-dose AZ regimen (e, f; AZ*5). In a control sample (a, b), right hemisphere, striatal (*), and hippocampal (arrow) atrophy along with cortical thinning (arrowhead, a) or cystic encephalomalacia (arrowhead, b) are again evident, similar to the LPS + HI pattern (compare with Fig. 2a, b). A representative AZ*3 sample (c, d) illustrates subtle right hemisphere tissue loss, striatal atrophy (*), and mild hippocampal atrophy together with pyramidal cell layer thinning (arrow). A representative AZ*5 sample (e, f) demonstrates mild right striatal (*) and hippocampal (arrow) tissue loss, but no evidence of cortical injury.
Panels a–f illustrate representative cresyl violet-stained coronal brain sections, obtained at P35, in samples from the three groups compared in Fig. 4. a–d P7 rats received injections of Pam3CSK4, followed by initiation 60 min HI (PAM + HI; see “Methods”). Two hours after the end of HI, animals started treatment with saline (a, b), the three-dose AZ regimen (c, d; AZ*3), or the five-dose AZ regimen (e, f; AZ*5). In a representative control sample (a, b), right hemisphere, striatal (*), and hippocampal (arrow) atrophy along with cortical thinning (arrowhead, a) or cystic encephalomalacia (arrowhead, b) are evident. A representative AZ*3 sample (c, d) illustrates subtle right hemisphere tissue loss, striatal atrophy (*), and mild hippocampal atrophy together with pyramidal cell layer thinning (arrow). A representative AZ*5 sample (e, f) demonstrates mild right striatal (*) and hippocampal (arrow) tissue loss, but no evidence of cortical injury. Scale bar = 2 mm.
Figure 6a–d summarizes results of experiments that evaluated the impact of the timing of treatment onset on AZ*5 efficacy in the PAM + HI model; P21 outcomes were compared after AZ treatment onset 1, 2, or 4 h after the end of HI, and in NS controls. Treatment initiated at 1 or 2 h after the end of HI improved outcomes with significantly higher median composite scores than saline controls (Fig. 6d, p < 0.001, Kruskal–Wallis); scores were not significantly higher than controls when treatment was delayed for 4 h. For all measures, there was a significant linear trend of decrement in AZ efficacy as delay to initiation increased from 1 h to 4 h vs. saline (p < 0.05, ANOVA post hoc linear test for trend).
Outcomes of AZ*5 regimens with initial-dose administration at 1, 2, or 4 h after the end of HI were compared with NS controls; outcomes were assessed at P21 (a–d) as in Fig. 3. AZ*5 treatment initiated at 1 or 2 h post HI, but not at 4 h, conferred improvements in the composite score (d) and sensorimotor test measures (a, b) (p < 0.005, Kruskal–Wallis; *p < 0.05 Dunn’s); %Intact right hemisphere was only significantly greater than controls for the 1 h delay group (c; *p < 0.05 Dunn’s). Overall, a linear decrement in AZ efficacy with increasing delay in treatment initiation from 1 to 4 h vs. NS was evident (p < 0.0001 post hoc linear test for trend).
To evaluate if there were sex differences in AZ efficacy, we incorporated both P35 and P21 composite scores (Supplementary Data Table S3). Composite scores differed between female and male controls (mean ± SD: female: 5.7 ± 6.3, male: 12.1 ± 1.3); increased numbers of deaths in females accounted for this difference. Modified composite scores (excluding deaths, which are assigned “0” in composite scores) were also calculated to distinguish treatment effects on survival and brain damage severity. Modified composite scores did not differ between sexes (female: 11.3 ± 1.5, male: 12.1 ± 1.3), i.e., in surviving animals sensorimotor deficits and brain damage did not differ. AZ*5 treatment was associated with increased survival in females (saline: 3/6 vs. AZ*5 9/10) and significantly improved both composite and modified composite scores in both sexes.
To further evaluate the relationship between sensorimotor function measures at P21 and P35, we incorporated results from all experiments in which performance was assessed in the same animals at both time points and analyzed the data in two ways (see Supplementary Data Table S4 and Supplementary Figure S1). In each model, for each test measure, results at P21 vs. P35 did not differ for any group, by two-way ANOVA factoring treatment and age, with Tukey’s multiple comparison test. In addition, comparing scores for each animal at the two ages (n = 64) revealed very strong relationships (simple linear regression: forepaw placing score: R2 = 0.84, p < 0.0001; L/R grip ratio: R2 = 0.54, p < 0.0001; see Supplementary Figure S1).
Discussion
In two complementary rodent models of inflammation-amplified neonatal HI brain injury, AZ treatment improved sensorimotor function and reduced brain damage. AZ treatment was more effective with longer durations (five doses over 4 days vs. three doses over 2 days) and with earlier onset of treatment (1 vs. 2 vs. 4 h) after the end of hypoxia exposure. These results are congruent with both our previous findings in the neonatal rodent HI model32 and reports in adult stroke and spinal cord injury models.33,41 Evidence of AZ-mediated neuroprotection in both straight-forward HI32 and inflammation-amplified HI models provide support for further evaluation of AZ as a potential treatment for NE.
Safety evaluation in both prior and current studies revealed no adverse effects of AZ treatment on core temperature, survival, or growth. More importantly, initial studies of AZ pharmacokinetics in critically ill premature infants enrolled in recent phase 1 and 2 clinical trials for the treatment of pulmonary disease revealed no safety concerns.28,30
Although composite measures are often used to compare neurodevelopmental outcomes in clinical trials, they have not been widely applied in experimental neuroprotection studies. We implemented a composite score calculation to integrate two quantitative measures of sensorimotor function with measures of brain damage. Although there were no discordant trends among the individual outcome measures, this novel approach readily delineated informative trends. An important feature of the composite score was that mortality was robustly integrated (assigned lowest possible score). Thus, the deleterious effects of any intervention that improved function and/or tissue preservation at the expense of increasing death would be evident. Although the present studies did not include cognitive performance measures, it would be feasible to incorporate selected quantifiable test measures in future studies. Composite scores yielded congruent outcomes when either % intact right hemisphere volumes (morphometry measures on P35) or right hemisphere weights (P21) were included.
Analysis of regional morphometry data did not reveal any selective vulnerability to inflammation-amplified injury in either model, and AZ reduced the extents of tissue damage in the cortex, striatum, hippocampus, and in other regions that encompass white matter tracts.
Since sex differences in injury mechanisms or treatment effects have been reported in some neonatal rodent HI studies,42,43,44,45 sex was systematically evaluated in these experiments. In control LPS + HI groups, we found no differences in survival or injury severity. In PAM + HI controls, we noted an unexpected higher mortality in females (although sample sizes were small), and, in fact, AZ treatment was associated with a higher survival rate. Moreover, in both models, AZ neuroprotective efficacy did not differ between female and male animals.
Of interest, other clinically available drugs can also attenuate LPS-amplified HI brain injury in neonatal rodents, when treatment is initiated up to 30 min after HI. These include the anti-inflammatory and free radical scavenger N-acetylcysteine (efficacy lost with a 2-h delay in treatment onset);46 the immunosuppressive sphingosine-1-phosphate receptor agonist fingolimod (begun 30 min after HI);47 and the immunosuppressant rapamycin (administered immediately after HI).48 Although no direct comparative efficacy studies among these drugs have been reported, the current data suggest that one advantage of AZ may be the longer duration of the therapeutic window for initiation of treatment than any of the other agents. As of March 2021, no reports have identified drugs that attenuate PAM-amplified HI brain injury, although immediate post-HI hypothermia is of benefit.37
This well-established neonatal rodent model of hypoxic–ischemic brain injury has inherent limitations that have been discussed in reviews.49,50 We relied on relatively simple, sensorimotor tests to assess functional outcomes; these tests are reliable, readily reproducible, and the supplementary data includes results to demonstrate that these tests identify deficits of similar magnitude over a two-week period (from P21 to P35).
We did not evaluate combined treatment with AZ and hypothermia, in view of the uncertainty of the translational relevance of the relatively brief (3–5 h) durations of hypothermia that are feasible experimentally in neonatal rats.51,52,53 Yet, the current results provide a strong impetus for future studies in larger animal models of neonatal inflammation-amplified asphyxial brain injury in which parenteral nutrition, physiologic monitoring, and much longer durations of hypothermia are feasible; data from neonatal piglets or fetal sheep would provide important complementary information about the safety and efficacy of AZ plus hypothermia combination treatment.54
Our report does not address the mechanism(s) of AZ neuroprotection in the pathophysiologically complex models of inflammation-amplified HI injury. AZ has well-characterized anti-inflammatory properties that are independent of its antibiotic activity, and multiple studies suggest that its primary cellular targets are macrophage and microglia.25 In adult CNS injury models, AZ neuroprotection is associated with the promotion of an M2 or pro-resolution macrophage/microglia phenotype.33,41 TLR2 and TLR4 are cell surface receptors with shared downstream intracellular signaling cascades that mediate both early pro-inflammatory and later regulatory and repair functions.55 We speculate that in both inflammation-amplified HI injury models AZ’s mode of action targets one or more shared downstream effector(s) of cell surface TLR signaling.
This report provides evidence of AZ’s potential efficacy as a treatment for NE. Equally important, our data raise important questions for future studies that will help clarify whether these findings support translation to early phase clinical trials. Priorities will include confirmation of safety and neuroprotective efficacy in complementary larger mammal models and in combination with clinically relevant hypothermia protocols.
References
Kurinczuk, J. J., White-Koning, M. & Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum. Dev. 86, 329–338 (2010).
Lee, A. C. et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr. Res. 74, 50–72 (2013).
Institute for Health Metrics and Evaluation. Institute for Health Metrics and Evaluation Findings from the Global Burden of Disease Study 2017 (Institute for Health Metrics and Evaluation, 2018).
Nelson, K. B. et al. Antecedents of neonatal encephalopathy in the vermont oxford network encephalopathy registry. Pediatrics 130, 878–886 (2012).
Aslam, S., Strickland, T. & Molloy, E. J. Neonatal encephalopathy: need for recognition of multiple etiologies for optimal management. Front. Pediatr. 7, 142 (2019).
McIntyre, S., Blair, E., Badawi, N., Keogh, J. & Nelson, K. B. Antecedents of cerebral palsy and perinatal death in term and late preterm singletons. Obstet. Gynecol. 122, 869–877 (2013).
Wu, Y. W. et al. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA 290, 2677–2684 (2003).
Rao, R. et al. Antimicrobial therapy utilization in neonates with hypoxic-ischemic encephalopathy (HIE): a report from the children’s hospital neonatal database (CHND). J. Perinatol. 40, 70–78 (2020).
Romero, R. et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J. Perinat. Med. 43, 19–36 (2015).
Romero, R. et al. Clinical chorioamnionitis at term X: microbiology, clinical signs, placental pathology, and neonatal bacteremia - implications for clinical care. J. Perinat. Med. 49, 275–298 (2021).
Sgro, M. et al. Early-onset neonatal sepsis: organism patterns between 2009 and 2014. Paediatr. Child Health 25, 425–431 (2020).
Polcwiartek, L. B. et al. Early-onset sepsis in term infants admitted to neonatal intensive care units (2011-2016). J. Perinatol. 41, 157–163 (2021).
Hagberg, H. et al. The role of inflammation in perinatal brain injury. Nat. Rev. Neurol. 11, 192–208 (2015).
Eklind, S. et al. Bacterial endotoxin sensitizes the immature brain to hypoxic-ischaemic injury. Eur. J. Neurosci. 13, 1101–1106 (2001).
Rice, J. E., Vannucci, R. C. & Brierley, J. B. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann. Neurol. 9, 131–141 (1981).
Towfighi, J., Yager, J. Y., Housman, C. & Vannucci, R. C. Neuropathology of remote hypoxic-ischemic damage in the immature rat. Acta Neuropathol. 81, 578–587 (1991).
Eklind, S., Arvidsson, P., Hagberg, H. & Mallard, C. The role of glucose in brain injury following the combination of lipopolysaccharide or lipoteichoic acid and hypoxia-ischemia in neonatal rats. Dev. Neurosci. 26, 61–67 (2004).
Lehnardt, S. et al. Activation of innate immunity in the CNS triggers neurodegeneration through a toll-like receptor 4-dependent pathway. Proc. Natl Acad. Sci. USA 100, 8514–8519 (2003).
Wang, X. et al. Lipopolysaccharide sensitizes neonatal hypoxic-ischemic brain injury in a MyD88-dependent manner. J. Immunol. 183, 7471–7477 (2009).
Wang, L. W., Chang, Y. C., Lin, C. Y., Hong, J. S. & Huang, C. C. Low-dose lipopolysaccharide selectively sensitizes hypoxic ischemia-induced white matter injury in the immature brain. Pediatr. Res. 68, 41–47 (2010).
Henneke, P. et al. Lipoproteins are critical tlr2 activating toxins in group B streptococcal sepsis. J. Immunol. 180, 6149–6158 (2008).
Mottahedin, A. et al. Systemic activation of toll-like receptor 2 suppresses mitochondrial respiration and exacerbates hypoxic-ischemic injury in the developing brain. J. Cereb. Blood Flow Metab. 37, 1192–1198 (2017).
Falck, M. et al. Hypothermic neuronal rescue from infection-sensitised hypoxic-ischaemic brain injury is pathogen dependent. Dev. Neurosci. 39, 238–247 (2017).
Falck, M. et al. Neonatal systemic inflammation induces inflammatory reactions and brain apoptosis in a pathogen-specific manner. Neonatology 113, 212–220 (2018).
Parnham, M. J. et al. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharm. Ther. 143, 225–245 (2014).
Shepard, R. M. & Falkner, F. C. Pharmacokinetics of azithromycin in rats and dogs. J. Antimicrob. Chemother. 25, 49–60 (1990).
Jaruratanasirikul, S., Hortiwakul, R., Tantisarasart, T., Phuenpathom, N. & Tussanasunthornwong, S. Distribution of azithromycin into brain tissue, cerebrospinal fluid, and aqueous humor of the eye. Antimicrob. Agents Chemother. 40, 825–826 (1996).
Viscardi, R. M. et al. Randomised trial of azithromycin to eradicate ureaplasma in preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 105, 615–622 (2020).
Viscardi, R. M. et al. Azithromycin to prevent bronchopulmonary dysplasia in ureaplasma-infected preterm infants: pharmacokinetics, safety, microbial response, and clinical outcomes with a 20-milligram-per-kilogram single intravenous dose. Antimicrob. Agents Chemother. 57, 2127–2133 (2013).
Merchan, L. M. et al. Pharmacokinetics, microbial response, and pulmonary outcomes of multidose intravenous azithromycin in preterm infants at risk for ureaplasma respiratory colonization. Antimicrob. Agents Chemother. 59, 570–578 (2015).
Hassan, H. E. et al. Pharmacokinetics, safety, and biologic effects of azithromycin in extremely preterm infants at risk for ureaplasma colonization and bronchopulmonary dysplasia. J. Clin. Pharm. 51, 1264–1275 (2011).
Barks, J. D. E., Liu, Y., Wang, L., Pai, M. P. & Silverstein, F. S. Repurposing azithromycin for neonatal neuroprotection. Pediatr. Res. 86, 444–451 (2019).
Amantea, D. et al. Azithromycin protects mice against ischemic stroke injury by promoting macrophage transition towards M2 phenotype. Exp. Neurol. 275(Part 1), 116–125 (2016).
Barks, J. D., Liu, Y. & Silverstein, F. S. Azithromycin reduces inflammation-amplified hypoxic-ischemic brain injury in neonatal rodents. Pediatr. Acad. Soc. Meeting Abstr. 4155, 4161 (2019).
Berman, D. R., Liu, Y. Q., Barks, J. & Mozurkewich, E. Docosahexaenoic acid confers neuroprotection in a rat model of perinatal hypoxia-ischemia potentiated by Escherichia coli lipopolysaccharide-induced systemic inflammation. Am. J. Obstet. Gynecol. 202, e461–e466 (2010).
Yang, D. et al. Plasminogen activator inhibitor-1 mitigates brain injury in a rat model of infection-sensitized neonatal hypoxia-ischemia. Cereb. Cortex 23, 1218–1229 (2013).
Falck, M. et al. Hypothermia is neuroprotective after severe hypoxic-ischaemic brain injury in neonatal rats pre-exposed to PAM3CSK4. Dev. Neurosci. 40, 189–197 (2018).
Felt, B. T. et al. Early appearance of functional deficits after neonatal excitotoxic and hypoxic-ischemic injury: fragile recovery after development and role of the NMDA receptor. Dev. Neurosci. 24, 418–425 (2002).
Barks, J. D. et al. Maternal high-fat diet influences outcomes after neonatal hypoxic-ischemic brain injury in rodents. J. Cereb. Blood Flow. Metab. 37, 307–318 (2017).
Andine, P. et al. Evaluation of brain damage in a rat model of neonatal hypoxic-ischemia. J. Neurosci. Methods 35, 253–260 (1990).
Gensel, J. C., Kopper, T. J., Zhang, B., Orr, M. B. & Bailey, W. M. Predictive screening of M1 and M2 macrophages reveals the immunomodulatory effectiveness of post spinal cord injury azithromycin treatment. Sci. Rep. 7, 40144 (2017).
Bona, E., Hagberg, H., Loberg, E. M., Bagenholm, R. & Thoresen, M. Protective effects of moderate hypothermia after neonatal hypoxia-ischemia: Short- and long-term outcome. Pediatr. Res. 43, 738–745 (1998).
Smith, A. L., Alexander, M., Rosenkrantz, T. S., Sadek, M. L. & Fitch, R. H. Sex differences in behavioral outcome following neonatal hypoxia ischemia: Insights from a clinical meta-analysis and a rodent model of induced hypoxic ischemic brain injury. Exp. Neurol. 254, 54–67 (2014).
Chavez-Valdez, R., Martin, L. J., Razdan, S., Gauda, E. B. & Northington, F. J. Sexual dimorphism in BDNF signaling after neonatal hypoxia-ischemia and treatment with necrostatin-1. Neuroscience 260, 106–119 (2014).
Wood, T. R. et al. Variability and sex-dependence of hypothermic neuroprotection in a rat model of neonatal hypoxic-ischaemic brain injury: a single laboratory meta-analysis. Sci. Rep. 10, 10833 (2020).
Wang, X. et al. N-acetylcysteine reduces lipopolysaccharide-sensitized hypoxic-ischemic brain injury. Ann. Neurol. 61, 263–271 (2007).
Yang, D. et al. Blocking lymphocyte trafficking with FTY720 prevents inflammation-sensitized hypoxic-ischemic brain injury in newborns. J. Neurosci. 34, 16467–16481 (2014).
Srivastava, I. N., Shperdheja, J., Baybis, M., Ferguson, T. & Crino, P. B. mTOR pathway inhibition prevents neuroinflammation and neuronal death in a mouse model of cerebral palsy. Neurobiol. Dis. 85, 144–154 (2016).
Northington, F. J. Brief update on animal models of hypoxic-ischemic encephalopathy and neonatal stroke. ILAR J. 47, 32–38 (2006).
Hamdy, N., Eide, S., Sun, H. S. & Feng, Z. P. Animal models for neonatal brain injury induced by hypoxic ischemic conditions in rodents. Exp. Neurol. 334, 113457 (2020).
Barks, J. D., Liu, Y. Q., Shangguan, Y. & Silverstein, F. S. Phenobarbital augments hypothermic neuroprotection. Pediatr. Res. 67, 532–537 (2010).
Osredkar, D. et al. Hypothermia is not neuroprotective after infection-sensitized neonatal hypoxic-ischemic brain injury. Resuscitation 85, 567–572 (2014).
Patel, S. D. et al. Therapeutic hypothermia and hypoxia-ischemia in the term-equivalent neonatal rat: characterization of a translational preclinical model. Pediatr. Res. 78, 264–271 (2015).
Zhou, K. Q., Davidson, J. O., Bennet, L. & Gunn, A. J. Combination treatments with therapeutic hypothermia for hypoxic-ischemic neuroprotection. Dev. Med. Child Neurol. 62, 1131–1137 (2020).
Luo, L., Lucas, R. M., Liu, L. & Stow, J. L. Signalling, sorting and scaffolding adaptors for toll-like receptors. J. Cell Sci. 133, jcs239194 (2019).
Acknowledgements
Research reported in this publication was supported by the Michigan Institute for Clinical & Health Research (MICHR) that is supported by the National Institutes of Health under award number UL1TR002240. The research was also supported by the National Institutes of Health under award number R21 HD096251 (to J.D.E.B. and F.S.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This research was also supported by the Reiter HIE Research Fund.
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: J.D.E.B., Y.L., I.A.D., and F.S.S. Drafting the article or revising it critically for important intellectual content: J.D.E.B. and F.S.S. Final approval of the version to be published: J.D.E.B., Y.L., I.A.D., and F.S.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Barks, J.D.E., Liu, Y., Dopp, I.A. et al. Azithromycin reduces inflammation-amplified hypoxic–ischemic brain injury in neonatal rats. Pediatr Res 92, 415–423 (2022). https://doi.org/10.1038/s41390-021-01747-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01747-5
This article is cited by
-
Comparing the efficacy in reducing brain injury of different neuroprotective agents following neonatal hypoxia–ischemia in newborn rats: a multi-drug randomized controlled screening trial
Scientific Reports (2023)
-
Follow-up study of infants recruited to the randomised, placebo-controlled trial of azithromycin for the prevention of chronic lung disease of prematurity in preterm infants—study protocol for the AZTEC-FU study
Trials (2022)