Abstract
Total parenteral nutrition (TPN) causes intrahepatic cholestasis and membrane phospholipid changes. Fatty acid (FA) composition of bile and hepatocyte phospholipid is influenced by dietary FA composition. We hypothesized that altering FA composition of i.v. lipid emulsions modifies 1) severity of TPN-induced cholestasis; 2) hepatocyte membrane composition and function; 3) bile flow and composition. Newborn piglets received either sow's milk, TPN with i.v. soybean oil or TPN with i.v. fish oil (FO). After 3 wk, basal and stimulated bile flow were measured after bolus injections of 20, 50, and 100 µmol/kg of taurocholate (TCA). Bile was analyzed for bile acids, cholesterol, phospholipids, and phospholipid-FA. Sinusoidal and canalicular membrane PL-FA, fluidity, and Na+/K+-ATPase were measured. Although the soybean oil-fed animals developed cholestasis, the FO and milk group had similar liver and serum bilirubin. Basal and stimulated bile flow rates were impaired in the soybean oil but not in the FO group. Hepatocyte membrane FA composition reflected dietary FA. Changes in sinusoidal and canalicular membrane fluidity and sinusoidal Na+/K+-ATPase activity did not explain the effect of FO on TPN-induced cholestasis. Intravenous FO reduces TPN-induced cholestasis by unknown mechanisms.
Similar content being viewed by others
Main
TPN-associated liver abnormalities are common in adults (1) and children (2) receiving long-term TPN. The development of TPN associated liver disease is particularly relevant in the premature newborn who is at significant risk of developing chronic liver disease (3).
Many factors have been implicated in causing TPN-associated liver disease (4). One of the components that has received little attention is the lipid composition of the emulsion used in TPN. In North America the most commonly used lipid emulsions are derived from soybean oil with a much higher linoleic acid content (5) than human milk or formula (6). One clinical report from the early 1980s has suggested a relationship between soybean-derived lipid and the development of cholestasis (7). No further investigation of this association has been published. However, in adult male African green monkeys, fish oil given by mouth has been shown to modify bile composition with an increase in biliary phospholipid secretion (8).
The n-3 fatty acids, such as those contained in fish oil, are important in the development of the neonatal central nervous system and retina (9), but their effect on hepatocyte function and bile flow has not been examined.
We have recently developed a model of TPN cholestasis in the newborn piglet (10). It was hypothesized that altering the fatty acid composition of i.v. lipid emulsions by using fish oil reduces the severity of TPN-induced cholestasis by preserving bile flow and composition that is due to modified hepatocyte membrane composition and function.
METHODS
Three groups of newborn piglets (Camborough/Canabreed) with birth weights between 1.2 and 2.0 kg entered the study. Group 1 (milk) was fed sow's milk. The animals in group 2 (SBO) and group 3 (FO) were fed i.v. for 19-20 d after entering the study at 36-48 h of age. TPN was given at 890 kJ/kg/d, and consisted of 31 kJ% as lipid, 40 kJ% as carbohydrate, and 29 kJ% as protein. The only difference in the feeding regimen between groups 2 and 3 was the composition of the lipid emulsion: group 2 was fed with a lipid emulsion prepared from 10% (wt/wt) SBO triglycerides (Sigma Chemical Co., St. Louis, MO), 1.2% egg yolk lecithin (Cal Biochem, San Diego, CA), 2.5% glycerol, and water, as described previously (10). The final fatty acid composition of this emulsion (Table 1) was similar to the fatty acid composition of lipid emulsions used in clinical practice. Group 3 was fed a lipid emulsion prepared from 10% (wt/wt) fish oil triglycerides (Nisshin Flour Milling Co., Nisshin, Japan), 1.2% egg yolk lecithin (Cal Biochem, San Diego, CA), 2.5% glycerol, and water (Table 1).
At entry, using general anesthesia, the i.v. fed animals had jugular catheters inserted bilaterally, advanced into the right atrium, and tunneled through an incision in the posterior neck. The animals were kept in a thermoneutral environment in plexiglass cages with swivels allowing freedom of movement and access to water ad libitum. A 10% amino acid solution (Travasol, Baxter, Toronto, Canada) was mixed with dextrose, multivitamins (1 mL/kg q2d; MVI-Pediatric, Rhone-Poulenc Rorer, Canada), essential trace elements (MTE-4, Lyphomed, Quebec, Canada), calcium gluconate (Abbott, Montreal, Canada), and electrolytes (11). This solution was infused via a metered pump infusion. This resulted in an intake of electrolytes and trace elements similar to those previously reported for TPN-fed animals (10,12). Compared with the previous report of Wykes and colleagues, zinc intake was half, and chromium and vitamin intake were twice as high in our study. The lipid emulsion was infused at 60 mL/kg/d. The amino acid/glucose solution and the lipid emulsion were infused in separate jugular catheters. The animals were weighed three times per week and plasma hemoglobin, electrolytes, and bilirubin concentrations were monitored weekly. The weight gain of each piglet was expressed as g per day per kg of birth weight, as calculated from weight at termination minus birth weight divided by age in days divided by birth weight. Approval of the Health Sciences Animal Welfare Committee of the University of Alberta was obtained for all procedures, according to the guidelines of the Canadian Council of Animal Care.
The control animals of the SBO and milk groups have previously been reported in a paper describing the use of i.v. ursodeoxycholic acid for preventing TPN-induced cholestasis. Due to the limited availability of equipment and personnel, a maximum of four animals could be studied simultaneously. Each animal was randomly assigned to one of several groups in an experiment that tested several different hypotheses and unrelated questions. Out if this one large experiment, some questions have been published previously (12), different hypotheses have been tested in this report, and other unrelated questions will be submitted shortly. Because more than 30 animals entered five different groups in parallel, and because these groups were not studied sequentially, it is justified to use the same SBO and milk groups as controls for the different studies that address different hypotheses and that are reported in different manuscripts.
Bile sampling. On the last day of the study, bile was collected and basal bile flow was measured in all animals. All studies were performed in the morning to control for possible circadian variation. In the i.v. fed animals, TPN was stopped 30 min before anesthesia, and all oral and i.v. fed animals received an infusion of 10% dextrose to replace fluid losses during the experiment. After intubation and under general anesthesia, the common bile duct, cystic duct, and gallbladder were identified through a midline abdominal incision. The cystic duct was ligated and the gallbladder contents were aspirated. Just proximal to its entry into the duodenum, the bile duct was carefully dissected, a polyethylene catheter was inserted, secured, and the abdomen was closed. The core temperature was monitored continuously with a rectal probe and maintained within 0.5°C of baseline. The entire procedure took 20-30 min, and bile collection was started approximately 1 h after the TPN was discontinued. Bile was collected in 10-min intervals in pre-weighed cryovials and the volume of the bile was measured gravimetrically assuming a density of 1. Basal flow rate was defined as three consecutive collections within 10% of each other.
Basal and stimulated bile flow. After basal bile flow had been established for three 10-min periods, each animal received 20 µmol/kg of TCA given i.v. over 10 min. Maximal stimulation was defined as the mean of the three greatest 10-min collection periods. Bile was collected until basal flow rate was achieved again. The animals were stimulated similarly with doses of 50 and 100 µmol/kg of TCA.
The TCA solution was prepared as a 100 mM stock solution by dissolving the sodium salt of TCA (Sigma) in saline after which it was sterilized through a 20-μ filter (Nalgene, NY). Other concentrations of TCA were made by dilution of the stock with sterile saline.
Liver and bile analysis. After completion of the bile collection, the piglet was killed by exsanguination and the liver was excised, rinsed with ice-cold saline, and weighed. A section of wet liver was preweighed, dried at 60°C for 24 h, and the dry liver weight was calculated as a percent of wet liver. The rest of the liver was cut in 30-g sections, wrapped in foil, and stored at -80°C. After Folch upper and lower phase extraction from liver homogenate (13), total bilirubin was measured spectrophotometrically at 450 nm using a molar absorptivity of 60,700 (14). Liver protein in the homogenate was determined by the Lowry method (15).
The total bile acid concentration of bile was determined by the 3-α hydroxysteroid method (16). Lipid was extracted from bile using the modified Folch procedure (13). Cholesterol and phospholipids were separated by thin layer chromatography on a silica gel G plate, using a petroleum ether:diethylether:glacial acetic acid 80:20:1 solvent system. Total cholesterol was measured by the O-phthaldehyde method (17). The phospholipid fraction was methylated with boron trifluoride-methanol, and was quantitated by gas liquid chromatography using diheptadeconoyl phosphatidylcholine as internal standard.
Membrane isolation and analysis. Using the technique of Ali (18), sinusoidal and canalicular membranes were isolated from a 30 g portion of liver. In a modification for piglet livers to increase membrane yield, the initial portion of liver was homogenized in a 1 mM NaHCO3 1.12 mM CaCl2 solution. The remainder of the technique including the discontinuous sucrose gradient and high speed centrifugation was performed as previously described (18). Membranes were prepared in triplicate, and analyses had less than 10% variability. The phospholipid, cholesterol, and phospholipid fatty acid compositions were determined by previously described techniques (9). No data on swine sinusoidal and canalicular membrane composition are available in either the medical or agricultural literature.
Membrane physical and enzyme properties. Membrane physical properties were assessed using a SLM-4800C spectrofluorometer. The lipid-soluble fluorescent probe 1,6-diphenyl-1,3,5-hexatriene was loaded into membrane vesicles using previously published methods (19). The final molar probe-to-lipid ratio approximated 1:1000. In this report the term "lipid fluidity" is used to denote the relative motional freedom of lipid molecules in the membrane bilayer. Probes such as 1,6-diphenyl-1,3,5-hexatriene have a relatively high limiting hindered anisotropy and, therefore, it has been suggested that membrane order plays an important role in determining the motional freedom of this probe. Thus, data from this probe can be used to estimate the static component of membrane fluidity (20). All data are reported as the steady-state anisotropy parameter. An increase in value of this parameter suggests a decrease in membrane lipid fluidity. The activity of membrane Na+/K+-ATPase was also determined (21).
Statistical analysis. All values are expressed as mean ± SEM. Basal bile flow was measured in 21 animals, 8 in group 1, 8 in group 2, and 5 in group 3. Of these animals, five animals in each group underwent bile flow stimulation. The six animals that did not undergo bile flow stimulation were part of an initial pilot study to determine basal bile flow variability over time, which was found to be less than 10% of the mean. The data of the same six animals were also used to estimate the sample size required to achieve statistical significance. For basal bile flow, using one-way ANOVA, making a conservative assumption of 100% difference between means with a SD of 40% of the means, and an α value of 0.05, a minimum sample size of five for each group was necessary to reach a power >0.8 (Sigmastat, Jandel Scientific, Rafael, CA). Differences between groups were analyzed using a one-way ANOVA with pairwise multiple comparisons by the Student-Newman-Keuls test (Sigmastat, Jandel Scientific, San Rafael, CA). When the normality or equal variance tests failed, a Kruskal-Wallis one-way ANOVA on ranks was used with Dunn's method for pairwise multiple comparisons. The effect of different TCA doses and diet were analyzed by two-way repeated measures ANOVA and all pairwise multiple comparisons by Student-Newman-Keuls test. Linear regression of bile acid output vs bile flow was used using the intercept to calculate BAIF and the slope for BADF. Multiple regression and partial F test were used to evaluate differences between slopes and intercepts. p < 0.05 was considered significant.
RESULTS
As outlined in "Methods," control animals of the milk and SBO groups have been reported previously in a study investigating the use of i.v. ursodeoxycholic acid to prevent TPN-induced cholestasis (12). This applies to data in Figures 1 and 2 and Tables 2, 3, and 6.
Basal and stimulated bile flow after 20, 50, and 100 µmol/kg of i.v. TCA. Except for 100 µmol/kg of TCA, bile flow in the orally fed (□) (n = 5) and FO group ( ) (n = 5) were similar. The bile flow increased significantly for each TCA dose in the milk-fed group, but no increases were noted in the SBO group (▪) (n = 5). At each dose, bile flow in the SBO group was lower than either in the milk-fed or FO group. At 100 µmol/kg of TCA, bile flow in FO group was higher compared with 0, 20, and 50 µmol/kg of TCA. Milk and SBO control groups have been reported previously (12).
) (n = 5) were similar. The bile flow increased significantly for each TCA dose in the milk-fed group, but no increases were noted in the SBO group (▪) (n = 5). At each dose, bile flow in the SBO group was lower than either in the milk-fed or FO group. At 100 µmol/kg of TCA, bile flow in FO group was higher compared with 0, 20, and 50 µmol/kg of TCA. Milk and SBO control groups have been reported previously (12).
Bile acid output vs bile flow in milk-fed (n = 5), SBO (n = 5), and FO (n = 5) animals. Both the BADF (slope; p < 0.05) and the BAIF (intercept; p < 0.05) were significantly reduced in the SBO (y = 0.91 + 4.90 x; r = 0.82) as compared with the milk fed (y = 6.17 + 8.24 x; r = 0.89) and FO (y = 5.29 + 7.85 x; r = 0.88) animals. There was no significant difference in slope or intercept in the milk fed vs FO group. Milk and SBO control groups have been reported previously (12).
No abnormalities were noted in serum electrolytes during the 3-wk study. Weight gain was similar in both groups of parenterally fed animals (SBO: 53.0 ± 2.5; FO: 50.9 ± 2.0 g/kg/d), but was lower than in the milk fed group (112.9 ± 9.4 g/kg/d; p < 0.05). There was, however, no difference between the wet liver weights (milk: 113.0 ± 4.7; SBO: 120.9 ± 3.5; FO: 125.6 ± 9.1 g/kg/d) and dry liver weights (milk: 24.9 ± 0.4; SBO: 23.9 ± 0.3; FO: 23.8 ± 0.3% of wet liver weight) at the time of death. Basal bile flow rate in the SBO group was significantly diminished compared with the milk-fed and FO animals (p < 0.05; Table 2). Although basal bile flow rate was significantly less in the FO compared with the milk-fed group, it was five times higher than in the SBO group (Table 2).
Bilirubin concentrations in serum and liver, serving as biochemical markers of cholestasis, were markedly elevated in the SBO group (123 ± 15.1 µmol/L and 4.01 ± 0.99 µmol/g protein) (p < 0.05) compared with the milk (3.8 ± 1.3 µmol/L and 0.22 ± 0.01 µmol/g protein) and the FO group (17.2 ± 2.4 µmol/L and 0.57 ± 0.12 µmol/g protein). As a consequence, biliary excretion of bilirubin in the SBO animal was reduced to less than 10% of the control group, and although excretion improved in the FO group, it was still half that in the milk group (Table 2). Bile acid, cholesterol, and phospholipid secretion were reduced in the SBO animals compared with the milk-fed animals. Although bile acid, cholesterol, and phospholipid secretion improved in the FO compared with the TPN group, they were not significantly different.
The changes in bile composition are shown in Table 3. In the TPN group compared with the two other groups, the molar composition of bile shows a decreased cholesterol saturation, measured as phospholipid plus bile acids divided by cholesterol (22). Fatty acid composition of the bile phospholipids reflected the fatty acids of the dietary fat (Table 4), with high n-6 and low n-3 content in the SBO and low n-6 and high n-3 content in the FO group. Similar diet-induced changes in fatty acid composition of phospholipids were found in sinusoidal and canalicular membranes (Table 5).
There were no differences in canalicular membrane fluidity or Na+/K+-ATPase activity among the three groups. The anisotropy parameter of the sinusoidal membranes was higher in both TPN groups, indicating lower fluidity (Table 6). The Na+/K+-ATPase activity in the sinusoidal membranes (Table 6) of the FO group was significantly lower than in the other groups.
Stimulation of bile flow with 20, 50, and 100 µmol/L of TCA per kilogram body weight resulted in significantly higher increases in bile flow for each respective dose in the milk-fed group (p < 0.05; Fig. 1). There was no difference in stimulated bile flow between the milk-fed and the FO group for 0, 20 and 50 µmol/kg of TCA. Despite the fact that the bile flow of the FO group at 100 µmol/kg of TCA was higher than for the lower doses, at the highest TCA dose the bile flow was significantly lower in the FO than in the milk-fed group (p < 0.05). Finally, the bile flow in the SBO group was markedly lower than in the milk-fed or FO group at all TCA doses (p < 0.05), and bile flow did not increase significantly with increasing TCA doses.
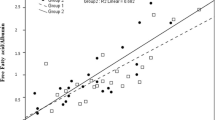
Both bile acid dependent component of bile flow (BAIF) and bile acid independent component of bile flow (BADF) were decreased in the SBO compared with the orally fed and FO animals (Fig. 2). Both intercept and slope were similar in the orally fed and FO group, yet the upper range of bile acid output was as high as 2.6 µmol/g liver/10 min in the milk-fed compared with 1.8 µmol/g liver/10 min in the FO group.
DISCUSSION
It has been previously demonstrated that the newborn piglet is a valid model for neonatal cholestasis (10,23,24). It has also been shown that physiologic indicators for cholestasis are more sensitive than histology (10,25,26). Yeh et al. (27) examined the effect of a lipid emulsion high in n-3 fatty acids on TPN-induced hepatic steatosis in rats and found a decreased hepatic triglyceride content compared with animals given i.v. lipid emulsion, high in eicosapentaenoic and docosahexaenoic acid and low in n-6 fatty acids, on the development of cholestasis. Newborn piglets fed TPN with i.v. FO as the lipid source did not develop cholestasis compared with animals fed the soy bean oil emulsion, which is similar to the clinically used products. This was supported by similar values for serum and liver bilirubin concentration, basal bile flow, and stimulated bile flow in FO and orally fed animals.
The effect of an i.v. FO emulsion on bile flow and TPN-induced cholestasis has not been studied previously. Although sow-fed piglets receive at least 1130 kJ/kg/day (11,28), a level that was achieved in short-term TPN studies (11), we elected to give 75-80% of the oral requirements, which is similar to the clinical situation of i.v.-fed infants who also receive three-fourths of the calories given to orally fed neonates (29). This accounts, at least in part, for the observed difference in weight gain between the i.v.- and milk-fed animals. Nevertheless, there was no difference in weight gain between the two i.v.-fed groups, and the wet and dry liver weights were similar in all groups.
We examined some aspects of the physiologic effect of an i.v. FO emulsion on bile flow; BADF and BAIF were similar in the FO and in the orally fed group, although both were decreased in the SBO group as previously demonstrated (10) (Fig. 2). Furthermore, bile flow stimulation with TCA is well preserved in the FO compared with the SBO group (Fig. 1). There are several mechanisms that could account for these observations. TPN has been associated with the formation of biliary sludge and stones that could contribute to the development of cholestasis (30). However, the composition of the bile macro-solutes cannot explain our findings. Using the (PL + BA)/C ratio (PL = phospholipids; BA = bile acids; C = cholesterol) as an index for lithogenicity (31), there is a significant decrease in cholesterol saturation in the SBO group but not in the FO group (Table 3). One of the factors recently shown to be important in cholesterol nucleation time and lipid secretion is the phospholipid fatty acid composition of bile (32). We have demonstrated a significant diet-induced change in fatty acid composition of biliary phospholipids, with n-3 fatty acids being predominant in the FO group (Table 4). Previously this has been linked with lower lithogenicity in orally fed monkeys (8).
A more likely explanation might be found in the effect of dietary lipid on alterations in membrane composition and function. Studies in various tissues of animals and humans have shown that membrane fatty acid composition is affected by dietary fatty acid composition (33). In i.v.-fed infants receiving an i.v. SBO emulsion, postmortem liver analysis showed a significant increase in phospholipid linoleic acid content compared with controls (34). Subcellular membrane analysis of fatty acid composition in hepatocyte phospholipids has not been done for i.v.-fed animals or humans. We have demonstrated that hepatic canalicular and sinusoidal membranes mirror the fatty acid composition of the i.v. lipid emulsions in this newborn piglet model for TPN (Table 5). Although this had little effect on the fluidity of the canalicular membranes, sinusoidal membrane fluidity was significantly different in both TPN groups compared with the orally fed group resulting in higher anisotropy. Therefore, as there is no difference between the two TPN groups, membrane lipid fluidity is unlikely to account for the preservation of bile flow in the FO group.
Fatty acid composition of membrane fatty acids can also influence membrane functions (35), such as the activity of membrane-bound enzymes. Sinusoidal Na+/K+-ATPase is required for the active transport of bile acids across this membrane (36,37). We found a decrease of about 50% in the activity of sinusoidal Na+/K+-ATPase in the FO as compared with the orally fed and SBO group. Although we have previously demonstrated that sinusoidal Na+/K+-ATPase activity is similar in sow's milk-fed and TPN-SBO-fed piglets (12), the decrease in activity in the FO group was unexpected. However, this process is not the rate-limiting step in bile acid transport to the canaliculus (38) and, therefore, the impairment of this enzyme in the FO animals may not be essential in reducing bile flow. Another possible explanation for the preservation of bile flow in FO animals may lie in the altered production of prostaglandins and eicosanoid metabolites. A recent study of bile secretion in the perfused rat liver has demonstrated a direct regulatory effect of series-2 prostaglandins on bile flow (39). In this study, prostaglandins F2α, D2, and E2 diminished bile flow and bile acid secretion, although infusion of prostaglandin inhibitors reversed this effect. We did not measure eicosanoids in our study, but it has been demonstrated previously, not only that administering large doses of linoleic acid increases series-2 prostanoid production (40,41) but also that a FO diet alters the type and amount of prostaglandins produced, resulting in lower levels of arachidonic acid and series-2 prostanoids (42–44). Either reduction in series-2 prostanoids and/or increase in series-3 prostanoids may account for our findings, and future studies in this area are needed.
In summary, an i.v. lipid emulsion high in eicosapentaenoic and docosahexaenoic acid reduces impairment of bile flow as seen in cholestasis caused by conventional TPN. Whereas hepatic canalicular and sinusoidal fatty acid composition of membrane phospholipids is significantly altered by the FO emulsion, it does not result in fluidity changes. NA+/K+-ATPase activity in sinusoidal membranes is diminished. Eicosanoid metabolites might be responsible for bile flow changes during TPN. It is also conceivable that the FO emulsion prevents cholestasis by a mechanism that counteracts another cause of the TPN-induced cholestasis. Further development and testing of FO-enriched emulsions are needed to determine the clinical application in parenteral nutrition for human neonates.
Abbreviations
- BA:
-
bile acids
- BADF:
-
bile acid dependent component of bile flow
- BAIF:
-
bile acid independent component of bile flow
- C:
-
cholesterol
- FA:
-
fatty acids
- PL:
-
phospholipids
- TCA:
-
taurocholate
- TPN:
-
total parenteral nutrition
References
Bashir RM, Lipman TO 1995 Hepatobiliary toxicity of total parenteral nutrition in adults. Gastroenterol Clin North Am 24: 1003–1025
Cohen C, Olsen MM 1981 Pediatric total parenteral nutrition. Liver histopathology. Arch Pathol Lab Med 105: 152–156
Beale EF, Nelson RM, Bucciarelli RL, Donnelly WH, Eitzman DV 1979 Intrahepatic cholestasis associated with parenteral nutrition in premature infants. Pediatrics 64: 342–347
Quigley EMM, Marsh MN, Shaffer JL, Markin RS 1993 Hepatobiliary complications of total parenteral nutrition. Gastroenterology 104: 286–301
Heard WC, Gomez MR 1993 Parenteral Nutrition. In: Tsang RC, Lucas A, Uauy R, Zlotkin S (eds) Nutritional Needs of the Preterm Infant: Scientific Basis and Practical Guidelines. Williams & Wilkins, Baltimore, 225–242.
Innis SM 1993 Fat. In: Tsang RC, Lucas A, Uauy R, Zlotkin S (eds) Nutritional Needs of the Preterm Infant: Scientific Basis and Practical Guidelines. Williams & Wilkins, Baltimore, pp 65: 86
Allardyce DB 1982 Cholestasis caused by lipid emulsions. Surg Gynecol Obstet 154: 641–647
Scobey MW, Johnson FL, Parks JS, Rudel LL 1991 Dietary fish oil effects on biliary lipid secretion and cholesterol gallstone formation in the African green monkey. Hepatology 14: 679–684
Hargreaves KM, Clandinin MT 1987 Phosphatidylethanolamine methyltransferase: Evidence for influence of diet fat on selectivity of substrate for methylation in rat brain synaptic plasma membranes. Biochim Biophys Acta 918: 97–105
Duerksen DR, Van Aerde JE, Chan G, Thomson ABR, Jewell L, Clandinin MT 1996 Total parenteral nutrition impairs bile flow and alters bile composition in the newborn piglet. Dig Dis Sci 41: 1864–1870
Wykes LJ, Ball RO, Pencharz PB 1993 Development and validation of a total parenteral nutrition model in the newborn piglet. J Nutr 123: 1248–1259
Duerksen DR, Van Aerde JE, Gramlich L, Meddings JB, Chan G, Thomson ABR, Clandinin MT 1996 Intravenous ursodeoxycholic acid reduces cholestasis in parenterally fed newborn piglets. Gastroenterology 111: 1111–1117
Folch J, Lees M, Stanley GHS 1957 A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226: 497–509
Lightner DA 1982 Bilirubin In: Heirwegh KP, Brown SB (eds) Chemistry, Vol 1. CRC Press, Boca Raton, FL, 36
Lowry OH, Rosebrough HJ, Farr AL, Randall RJ 1951 Protein measurement with the folin phenol reagent. J Biol Chem 193: 265–275
Ross FW, Mayer D, Haindl H 1974 Bile Acids. In: Bermeyer HU (ed) Method of Enzymatic Analysis, Vol 4. Academic Press, Orlando, FL, 1886–1889.
Rudell I, Morris MD 1973 The determination of cholesterol in lipid extracts with O-phthaldehyde. J Lipid Res 14: 364–366
Ali N, Alique R, Evans WH 1990 Highly purified bile-canalicular vesicles and lateral plasma membranes isolated from rat liver on Nycodenz gradients. Biochem J 271: 185–192
Meddings JB, Theisen S 1989 Development of rat jejunum:lipid permeability, physical properties and chemical composition. Am J Physiol 256: G931–G940
Schacter D 1984 Fluidity and function of the hepatocyte plasma membranes. Hepatology 4: 140–151
Verity MA 1972 Cation modulation of synaptosomal respiration. J Neurochem 19: 1305–1317
Gimmon Z, Kelley RE, Simko V, Fischer JE 1982 Total parenteral nutrition solution increases bile lithogenicity in rat. J Surg Res 32: 256–263
Shulman RJ, Fioroto ML, Sheng HP, Finegold MJ, Garza C 1987 Liver composition and histology in growing infant miniature pigs given different total parenteral nutrition fuel mixes. JPEN 2: 275–279
Truskett PG, Shi CP, Rose M, Sharp PA, Ham JM 1987 Model of TPN-associated hepatobiliary dysfunction in the young pig. Br J Surg 74: 639–642
Cohen IT, Meunier KM, Lipman RD, Ellis NG 1986 Spectrum of hepatic, splenic and pulmonary histopathology in the hyperalimented neonatal piglet. In: Tumbleson ME, (ed) Swine in Biomedical Research. Plenum Press, New York, 1253–1263.
Simon FR 1994 Physiology and pathophysiology of bile secretion. In: T Gitnick (ed) Principles and Practice of Gastroenterology and Hepatology. Appleton & Lange, Norwalk, CT, 723–730.
Yeh SL, Chen WJ, Huang PC 1996 Effects of fish oil and safflower oil emulsions on diet-induced hepatic steatosis in rats receiving total parenteral nutrition. Clin Nutr 15: 80–83
National Research Council 1988 Nutrient Requirements of Swine, 9th ed. National Academic Press, Washington, DC, 49–53.
Putet G 1993 Energy. In: Tsang RC, Lucas A, Uauy R, Zlotkin S, (eds) Nutritional Needs of the Preterm Infant: Scientific Basis and Practical Guidelines. Williams & Wilkins, Baltimore, 15–28.
Messing B, Bories F Kuntstlinger, Bernier JJ 1983 Does total parenteral nutrition induce gallbladder sludge formation and lithiasis? Gastroenterology 84: 1012–1019
Gimmon Z, Kelley RE, Simko V, Fischer JE 1982 Total parenteral nutrition solution increases bile lithogenicity in rat. J Surg Res 32: 256–263
Berr FR, Holl J, Jungst D, Fischer S 1992 Dietary N-3 polyunsaturated fatty acids decrease biliary cholesterol saturation in gallstone disease. Hepatology 16: 960–967
Van Aerde JE, Clandinin MT 1993 Controversy in fatty acid balance. Can J Physiol Pharmacol 71: 707–712
Martinez M, Ballabriga A 1987 Effects of parenteral nutrition with high doses of linoleate on the developing human liver and brain. Lipids 22: 133–138
Clandinin MT, Cheema BS, Field CJ, Baracos VE 1993 Dietary lipids influence lin action. Ann NY Acad Sci 683: 151–163
Inoue M, Kinne R, Tran T 1982 Taurocholate transport by rat liver sinusoidal membrane vesicles: Evidence for sodium co-transport. Hepatology 2: 572–579
Van Dyke RW, Stephens JE, Scharschmidt BF 1982 Bile acid transport in cultured rat hepatocytes. Am J Physiol G484–G492
Nathanson MH, Boyer JL 1991 Mechanisms and regulation of bile secretion. Hepatology 14: 551–566
Beckh K, Kneip S, Arnold R 1994 Direct regulation of bile secretion by prostaglandins in perfused rat liver. Hepatology 19: 1208–1213
Epstein M, Lipschitz M, Rapport K 1982 Augmentation of prostaglandin production by linoleic acid in man. Clin Sci 863: 565–571
Hunt CV, Pachman L, Hageman J, Cobb, MA, Klemka L 1986 Liposyn infusion increases plasma prostaglandin concentrations. Pediatr Pulmonol 2: 154–158
Lorenz R, Spengler U, Fisher S, Duhm J, Weber PC 1983 Platelet function, thromboxane formation and blood pressure control during supplementation of the western diet with cod liver oil. Circulation 67: 504–511
Archer S, Johnson G, Gebhard R, Castleman WL, Levine AS, Westcott JY, Voelkel NF, Nelson DP, Weir EK 1989 Effect of dietary fish oil on lung lipid profile and hypoxic pulmonary hypertension. J Appl Physiol 66: 1662–1673
Robinson D, Prickett J, Polisson R 1985 The protective effect of dietary fish oil on marine lupus. Prostaglandins 30: 51–75
Acknowledgements
The authors thank E. Paslawski and A. Wierzbicki for technical assistance and J. Minckler for secretarial contribution. MTE-conc trace elements were kindly donated by Lyphomed, Canada, calcium gluconate by Abbott, Canada, and MVI-Pediatric by Rhone-Poulenc Rorer, Canada. Drs. D. Duerksen and L. Gramlich were recipients of Glaxo Research Fellowships awarded through the Canadian Association of Gastroenterology.
Author information
Authors and Affiliations
Additional information
Supported by grants from the Medical Research Council of Canada, Alberta Heritage Foundation for Medical Research and Northern Alberta Children's Health Foundation.
Rights and permissions
About this article
Cite this article
Van Aerde, J., Duerksen, D., Gramlich, L. et al. Intravenous Fish Oil Emulsion Attenuates Total Parenteral Nutrition-Induced Cholestasis in Newborn Piglets. Pediatr Res 45, 202–208 (1999). https://doi.org/10.1203/00006450-199902000-00008
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199902000-00008
This article is cited by
-
Fish oil-containing lipid emulsions prevention on parenteral nutrition-associated cholestasis in very low birth weight infants: a meta-analysis
World Journal of Pediatrics (2022)
-
Intestinal Rehabilitation
Current Surgery Reports (2015)
-
Comparison of liver function with two new/mixed intravenous lipid emulsions in children with intestinal failure
European Journal of Clinical Nutrition (2014)
-
Parenteral Lipid Emulsions in Guinea Pigs Differentially Influence Plasma and Tissue Levels of Fatty Acids, Squalene, Cholesterol, and Phytosterols
Lipids (2014)
-
Fish Omega-3 Fatty Acids Induce Liver Fibrosis in the Treatment of Bile Duct-Ligated Rats
Digestive Diseases and Sciences (2013)