Abstract
Adrenarche is the increase of adrenal androgen secretion, mainly dehydroepiandrosterone and dehydroepiandrosterone sulfate, that occurs during prepuberty in higher primates. This event takes place at about 6-8 y of age in humans. It had been postulated that adrenarche might reflect an increase in the 17,20 lyase:17OH-ase activity ratio of microsomal cytochrome P450c17. However, studies to demonstrate this mechanism have been unsuccessful. Because it has been described that virilizing adrenocortical carcinomas have high dehydroepiandrosterone sulfate secretion and low 3β-hydroxysteroid dehydrogenase (3βHSD) activity, in this study we evaluated the possible existence of maturative changes of the level of 3βHSD type II mRNA in 11 normal prepubertal and early pubertal human adrenals. Adrenal glands from subjects aged 0.1 to 13 y were obtained from organ donors, patients undergoing resection of the kidney for renal neoplasms or necropsies with less than 6 h of postportem time. The expression of 3βHSD type II gene was studied by dot blot in all samples and by relative reverse transcriptase (RT)-PCR in nine samples. The size of the transcripts was evaluated by Northern blot. Hybridization was performed using labeled human 3βHSD cDNA probes. The uniformity of loading was tested using labeled human βactine cDNA. The relative intensities of hybridization signals were quantified by scanning densitometry. The expected bands after relative RT-PCR were eluted, and radioactivity was measured in a scintillation counter. For the analysis of the results, subjects were divided into two groups as a function of age: group 1, less than 8 y (n = 6; range 0.1-2.48 y) and group 2, equal or older than 8 y (n = 5; range 8-13 y). 3βHSD type II mRNA expression, analyzed by dot blot and relative RT-PCR, was significantly higher (p < 0.05) in group 1 (median and range 4.99, 0.50-8.00 and 16.3, 13.5-40.0 arbitrary units, respectively) than in group 2 (0.15, 0.12-0.75 and 5.66, 3.18-13.0, respectively). In conclusion, we have shown a decrease of the expression 3βHSD type II gene as a function of age in prepubertal and early pubertal normal human adrenal tissue. This maturative change might be involved in the mechanism of human adrenarche.
Similar content being viewed by others
Main
Adrenarche is an event of postnatal sexual maturation in which there is an increase in the secretion of adrenal androgens, principally DHEA and DHEA sulfate, not accompanied by an increase in cortisol secretion. This event occurs only in higher primates, typically at about 6-8 y of age in humans(1–3). The mechanism of this phenomenon is not known, but it is independent of the gonadotropin-releasing hormone pulse generator activation at sexual maturation. The initial step of the steroidogenic pathway is the side chain cleavage of cholesterol, catalyzed by cytochrome P450 side chain cleavage to form pregnenolone(4), which might be converted to progesterone by 3βHSD type II, the isoform present in human adrenals and gonads. In the adrenal cortex, the subsequent 17α hydroxylation of pregnenolone or progesterone is a branch point for the formation of cortisol and adrenal androgens. 17α-hydroxylase and 17,20 lyase activities are catalyzed by microsomal cytochrome P450c17, a product of a single copy gene CYP17. The 17,20 lyase activity cleaves the c17,20 bond to convert the 21-carbon steroid 17OH pregnenolone to the corresponding 19-carbon steroid DHEA(5,6). Whether the 17,20 lyase activity of human P450c17 can convert 17OH-progesterone to androstenedione is not clearly established(7–9). In addition, secretion of cortisol and androgens is dissociated in many clinical situations(7–10). Although little is known about the mechanism of this dissociation, it has been suggested that the electron transfer system might be a clue, as the flux of reducing equivalents to P450c17 might be crucial to regulate 17,20 lyase activity(11–13). However, a decrease of 3βHSD type II mRNA with normal P450c17 mRNA and a normal ratio of 17,20 lyase to 17α-hydroxylase activities has been found in virilized adrenocortical carcinomas, suggesting little contribution of the electron transfer system to the modulation of 17,20 lyase activity(14). These authors speculated that the high androgen secretion observed in patients with adrenocortical carcinomas might be secondary to a marked reduction in 3βHSD activity. Therefore, we have hypothesized that a progressive decrease in 3βHSD activity as a function of age during the prepubertal years could be a mechanism of the increment of human adrenal DHEA production at adrenarche. Therefore, in this study we have examined the existence of maturative changes in the expression of the 3βHSD type II gene. To this end, we have studied the abundance of 3βHSD mRNA in 11 specimens of normal human adrenal tissue obtained from prepubertal and early pubertal subjects.
MATERIALS AND METHODS
Clinical Material
Adrenal glands from subjects aged 0.1 to 13 y were obtained from organ donors, patients undergoing resection of the kidney for renal neoplasms or necropsies with less than 6 h of post-mortem time (Table 1). Immediately after adrenal gland removal, all samples were stored at -190°C for mRNA analysis. The study was approved by the Research Committee of the Garrahan Pediatric Hospital.
For analysis of the results, subjects were divided into two groups as a function of age: group 1, less than 8 y old (n = 6; range 0.1 to 2.48 y) and group 2, equal or older than 8 y old (n = 5; range, 8 to 13 y). This division was based on the mean age of adrenarche reported in normal human subjects(2).
Preparation of RNA
Total RNA was isolated from tissues by homogenizing tissue samples in the presence of 1 mL of trizol reagent (Life Technologies, Inc.) per 50-100 mg of tissue, according to the manufacturer's instructions. Extraction was carried out in the presence of 0.2 mL of chloroform per 1 mL of trizol reagent. Samples were centrifuged at 12 000 × g for 15 min at 4°C. RNA was precipitated from the aqueous phase by mixing with 0.5 mL of isopropyl alcohol at -20°C for 2 h. The RNA pellet was washed with 1 mL of 75% ethanol, dissolved in RNAase free water, and stored at -85°C.
RNA Analysis
In most samples, 3βHSD type II mRNA was analyzed by do not and by relative RT-PCR. The size of the 3βHSD type II mRNA transcript was examined by Northern blot in three samples.
Dot blot. The method of Voutilainen et al.(15) was followed for dot blot analysis. RNA samples were denatured in 7.5% formaldehyde and 6 × SSC (0.9 M NaCl, 0.09 M sodium citrate) at 60°C for 30 min and then spotted on nitrocellulose filters (BA 85, Schleicher and Schuell). Filters were baked for 2 h at 80°C and prehybridized in buffer containing 50% formamide, 6 × SSC, 0.1% Ficoll, 0.1% BSA, 0.1% polyvinyl pyrrolidone, 100 µg/mL salmon sperm DNA, and 100 µg/mL yeast RNA for 4 h at 42°C before probe was added. Hybridization was performed using labeled human placental 3βHSD cDNA (kindly supplied by Dr. F. Labrie)(16). Probes were labeled in the presence of deoxy-[α-32P]CTP, using the technique described by Feinberg and Vogelstein(17). After hybridization, the filters underwent four 20-min washes in 0.2 × SSC, 0.1% SDS at 50°C, before autoradiography at -70°C for 24 h. Dots for all samples studied were analyzed on a single film. The uniformity of loading was tested using a labeled human βactin cDNA probe generated by PCR using the following primers: 5′ GGACCTGACTGACTACCTCATGAA 3′ and 5′ GATCCACATCTGCTGGAAGGTGG 3′ (a 524-bp amplified fragment from exon 3 to exon 5). The relative 3βHSD/βactin intensities of hybridization signals (arbitrary units) were quantified by scanning densitometry.
RT-PCR. First strand of cDNA was synthesized using Molony murine leukemia virus reverse transcriptase (Pro-mega). One to 5 µg of total RNA was incubated at 37°C for 60 min, with 200 U of enzyme, 1 × reaction buffer, 20-40 U of rRNAsin ribonuclease inhibitor, 200 ng of oligo-dt, and 10 mM of each deoxynucleotide triphosphate. The exponential phase for both 3βHSD type II and βactin PCRs was standardized using placental RT-derived cDNA at different cycles. It was found that 20 cycles corresponded to the exponential phase in the two PCRs. Relative PCR was performed in replicates using two dilutions of the RT product as template. 3βHSD and βactin cDNA were coamplified at the exponential phase (20 cycles) in the same tube, using specific primers. The βactin primer was prepared as described previously(18). Amplification of a 421-bp fragment (from bp +36 to +457) of the 3βHSD type II gene was performed using the following primers: 5′ GGGCTGGAGCTGCCTTGTGA 3′ and 5′ TCGTGGCGTTCTGGATGAT 3′ PCR was carried out in a 25-µL mixture containing 10 × reaction buffer, 2.5 µL of MgCl2 (25 mM), 2.5 µL of DMSO, 1 µL of deoxynucleotide triphosphate mixture (1.25 mM each one), 4 µL of 0.05 µM of each forward and reverse βactin primers, 0.5 µM of each forward and reverse 3βHSD type II primers, and 1.5 U of Taq polymerase. The 5′ ends of forward primers were labeled with [γ-32P]ATP by T4 polynucleotide kinase (Promega) before PCR amplification. Labeled PCR products were resolved by electrophoresis on 6% denaturing polyacrylamide gels. The expected bands were visualized by autoradiography, and radioactivity was measured in a β-scintillation counter. The 3βHSD/βactin ratio was calculated for every sample. Measurements were compared at two dilutions to confirm that values were quantitatively the correct ones as expected in the exponential phase.
Northern blot. The method of Sambrook et al.(19) was followed for Northern blot analysis. About 15 µg of RNA was denatured in a buffer containing 10% formaldehyde and 50% formamide and electrophoresed on 10% formaldehyde, 1% agarose gel. RNA from these gels was transferred to a nitrocellulose membrane, and the blot was baked and prehybridized as described above for dot blots. Autoradiography was carried out at -70°C for 5 d. As expected, a 1.7-kb band(20) was found for the 3βHSD type II transcript (Fig. 1, lower panels).
Upper panels, autoradiography of dot blots after successive hybridization (and stripping) with βactin and 3βHSD type II. From a3 to d1, both for 3βHSD type II (left) and βactin (right), dots correspond to adrenal RNA from the 11 patients, arranged by age from the youngest to the oldest, from left to right in the four rows (d2, placental tissue; d3, normal adult adrenal; d4, normal peripheral leukocytes). Middle panel, 6% PAGE of cDNA-amplified products after relative RT-PCR in one subject of group 1 and in two subjects of group 2. Lanes 1-4, adrenal tissue from a 0.56-year-old subject at two dilutions in duplicate; lanes 5-7, adrenal tissue from a 9-year-old subject at two dilutions, single sample (lane 5) and duplicates (lanes 6 and 7). Lane 8, negative control (no template). Lanes 9-12, adrenal tissue from an 11-year-old subject at two dilutions, in duplicate. Lower panels, Northern blot of 3βHSD type II (left) and βactin (right) mRNA in three patients, aged 0.1, 0.56, and 1.64 y.
To check if the expression of βactin mRNA in human prepubertal adrenal tissue is age dependent, Northern blots analysis, using 7 µg of total RNA and a labeled human βactin cDNA probe, was carried out in four samples of group 1 and in three samples of group 2. Data were normalized to ethidium bromide-stained 18 S ribosomal RNA. To this effect, the relative βactin/18 S intensities were determined using a Molecular Image System (Bio-Rad GS-505). Means (±SD) were 0.35 ± 0.03 in group 1 and 0.28 ± 0.06 AU in group 2 (p not significant).
Statistical Analysis
All 3βHSD type II m RNA values are reported as median and range. Data were analyzed using the Mann-Whitney's test. p < 0.05 was considered statistically significant.
RESULTS
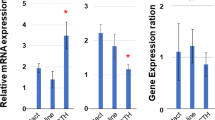
Measurement of 3βHSD type II mRNA by dot blot in groups 1 and 2 is shown in Table 1 and in Figure 1 (upper panels). For a valid comparison, RNA samples from adrenal tissues obtained from the 11 subjects were studied using the same probes in the same blot. Median 3βHSD type II mRNA in group 1 was significantly higher than in group 2 (p < 0.05).
Results of relative RT-PCR for every sample is also shown in Table 1. Two patients could not be studied with this method. Similarly to what was observed by dot blot, the median of group 1 was significantly higher than that of group 2 (p < 0.05). Examples of 6% PAGE of amplified cDNAs in three subjects are shown in Figure 1 (middle panel).
DISCUSSION
The data presented here show, by two different methods, that in human adrenal tissue the 3βHSD type II mRNA transcript in the young prepubertal group is significantly higher than in the old prepubertal and early adolescent group. Even though we have determined abundance of 3βHSD type II mRNA, it is assumed that abundance reflects gene expression rather than degradation of the transcript. This finding might be related to the mechanism of adrenarche. At adrenarche, the human adrenal cortex begins to secrete significant quantities of Δ5 C19 steroids, suggestive of an altered regulation of 3βHSD type II activity relative to other enzymes of the steroidogenic pathway, particularly cytochrome P450c17. It has been suggested that the adrenal 3βHSD expression in humans may be the critical regulatory step governing production of the various C21 and C19 steroid hormones(21,22).
The regulation and function of the primate fetal adrenal gland has been a subject of considerable interest(23). The fetal zone of the human fetal adrenal has a remarkable capacity to produce very large amounts of DHEA and minimal cortisol, suggesting a major selective block of either 3βHSD type II enzyme activity or gene expression during fetal life. Indeed, it was established that the massive secretion of Δ5 C19 steroids from the fetal zone of fetal adrenal occurred because of the absence of 3βHSD type II gene expression and not inhibition of 3βHSD type II activity(24). The nature of the suppression of the 3βHSD type II gene in fetal adrenal has to be elucidated. Studies of functional zonation have established that the fetal zone of the fetal adrenal is the site of Δ5 steroid production and of repression of the expression of the 3βHSD type II gene(25).
In adult human adrenal cortex, the zona reticularis is the site of biosynthesis of DHEA and DHEA sulfate resulting from the low gene expression of 3βHSD type II mRNA. The age-related decline in adult adrenal androgen biosynthesis might be secondary to an age-related decline in the number of functional reticularis cells(26). Furthermore, in a previous study, Gell et al.(27) have reported an age-dependent decrease in immunohistochemically localized 3βHSD in adrenal zona reticularis during prepuberty and have suggested that this decrease may contribute to the increased production of DHEA and DHEA sulfate seen at adrenarche.
Adrenal steroidogenesis might be under the influence of paracrine regulators. It has been reported that the effect of TGFβ1 in cultured human fasciculata reticularis cells is not to reduce basal or ACTH-stimulated cortisol production but to decrease the production of DHEA sulfate(28). These authors postulated that a local diminution of TGFβ1 might be involved in the mechanism of adrenarche.
On the other hand, it has been suggested that adrenarche might be secondary to an increment of 17,20 lyase activity by a regulatory effect independent of 17 α-hydroxylase activity. It has been suggested that an unidentified intra-adrenal event influencing the molar abundance of redox partners(11–13) rather than the level of P450c17 mRNA, protein abundance, or P450 oxidoreductase abundance would be responsible for the regulatory shift. In addition, it has been hypothesized that the regulation of 17,20 lyase activity was mediated by posttranslational modification of P450c17 by serine threonine phosphorylation in response to a cAMP-dependent mechanism. This would increase the efficiency of electron transfer secondary to an increment in the affinity of P450c17 for P450 oxidoreductase(29).
3βHSD type II is an NAD+ membrane-bound enzyme, but its intracellular localization remains controversial because the enzyme has been located in the microsomal and in the mitochondrial fractions of adrenal tissue(30–33). In this respect, and because little is known about the anchoring of 3βHSD to the membrane, an interaction between the decrement of 3βHSD activity and an increment of 17,20 activity cannot be ruled out.
In summary, our data indicate that a decrease in the activity of 3βHSD type II enzyme, secondary to an inhibition in the expression of the 3βHSD type II gene in adrenal cells, is an important underlying mechanism in the increase of DHEA secretion characteristic of adrenarche.
Abbreviations
- DHEA :
-
dehydroepiandrosterone
- P450c17 :
-
cytochrome P450c17
- 3βHSD :
-
3β-hydroxysteroid dehydrogenase
- RT :
-
reverse transcriptase
References
Sklar CA, Kaplan SL, Grumbach MM 1980 Evidence for dissociation between adrenarche and gonadarche: studies in patients with idiopathic precocious puberty, gonadal dysgenesis, isolated gonadotropin deficiency, and constitutionally delayed growth and adolescence. J Clin Endocrinol Metab 51: 548–556.
Parker LN 1991 Adrenarche. Endocrinol Metab Clin North Am 20: 71–78.
Migeon CJ, Keller AR, Laurence B, Shepard THI 1957 Dehydroepiandrosterone and androsterone levels in human plasma: effect of age and sex, day to day and diurnal variations. J Clin Endocrinol Metab 17: 1051–1056.
Miller WL 1988 Molecular biology of steroid hormone synthesis. Endocr Rev 9: 295–318.
Nakajin S, Shivley JE, Yuan P, Hall PF 1981 Microsomal cytochrome P450 from neonatal pig testis: two enzymatic activities (17 α-hydroxylase and c 17:20 lyase) associated with one protein. Biochemistry 20: 4037–4042.
Zuber MX, Simpson ER, Waterman MR 1986 Expression of bovine 17 hydroxylase cytochrome P450 cDNA in non-steroidogenic (COS 1) cells. Science 234: 1258–1260.
Fevold HR, Lorence MC, McCarthy JL, Trant JM, Kagimoto M, Waterman MR, Mason JI 1989 Rat P 450-17α from testis characterization of a full-length cDNA encoding a unique steroid hydroxylase capable of catalyzing both δ4- and δ5- steroid-17,20 lyase reactions, Mol Endocrinol 3: 968–975.
McAllister JM, Kevin JFP, Trant JM, Estabrook RW, Mason JI, Waterman MR, Simpson ER 1989 Regulation of cholesterol side-chain cleavage and 17α-hydroxylase/lyase activities in proliferating human theca interna cells in long-term monolayer culture. Endocrinology 125: 1959–1966.
Lin D, Harikrishna JA, Moore CCD, Jones KL, Miller WL 1991 Missense mutation Ser106→Pro causes 17α-hydroxylase deficiency. J Biol Chem 266: 15992–15998.
Parker LN, Odell WD 1980 Control of adrenal androgen secretion. Endocr Rev 1: 392–410.
Onoda M, Hall PF 1982 Cytochrome b5 stimulates purified testicular microsomal cytochrome P-450 (C21 side-chain cleavage). Biochem Biophys Res Commun 108: 454–460.
Shinzawa K, Kominami S, Takemori S 1985 Studies on cytochrome P450 (P45017α, lyase) from guinea pig adrenal microsomes: dual function of a single enzyme and effect of cytochrome b5. Biochim Biophys Acta 833: 151–160.
Lin D, Black SM, Nagahama Y, Miller WI 1993 Steroid 17α-hydroxylase and 17,20 lyase activities of P450c17: contributions of serine 106 and P450 reductase. Endocrinology 132: 2498–2506.
Sakai Y, Yanase T, Hara T, Takayanagi R, Haji M, Nawata H 1994 Mechanism of abnormal production of adrenal androgens in patients with adrenocortical adenomas and carcinomas. J Clin Endocrinol Metab 78: 36–40.
Voutilainen R, Tapanainen J, Chung B-Ch, Matteson KJ, Miller WL 1986 Hormonal regulation of P450scc ( 20,22-desmolase) and P450c17 (17α-hydroxylase/17,20-lyase) in cultured human granulosa cells. J Clin Endocrinol Metab 63: 202–207.
Luu The V, Lachance Y, Labrie C, Leblanc G, Thomas JL, Strickler RC, Labrie F 1989 Full length cDNA structure and deduced aminoacid sequence of human 3β-hydroxy-5-ene steroid dehydrogenase. Mol Endocrinol 3: 1310–1312.
Feinberg AP, Vogelstein B 1984 A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity (addendum). Anal Biochem 137: 266–267.
Peng C, Huang Th, Yeung E-B, Donaldson CJ, Vale WW, Leung PCK 1993 Expression of the type II activin receptor gene in the human placenta. Endocrinology 133: 3046–3049.
Sambrook J, Fritsch EF, Maniatis T 1989 Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 46–52.
Rheaume E, Lachance Y, Zhao HF, Breton N, Dumont M, Launoit Y, Trudel C, Luu-The V, Simard J, Labrie F 1991 Structure and expression of a new complementary DNA encoding the almost exclusive 3-hydroxy steroid dehydrogenase/5-4-isomerase in human adrenals and gonads. Mol Endocrinol 5: 1147–1157.
McAllister JA, Hornsby PJ 1988 Dual regulation of 3β-hydroxysteroid dehydrogenase, 17α-hydroxylase, and dehydroepiandrosterone sulfotransferase by adenosine 3′,5′-monophosphate and activators of protein kinase C in cultured human adrenocortical cells. Endocrinology 122: 2012–2018.
Cheung CY, Flasch MV, Hornsby PJ 1992 Expression of 17α-hydroxylase and 3β-hydroxysteroid dehydrogenase in fetal human adrenocortical cells transfected with SV40 T antigen. J Mol Endocrinol 9: 7–17.
Jaffe RB, Serrón-Ferré M, Crickard K, Koritink D, Mitchell BF, Huhtaniemi IT 1981 Regulation and function of the primate fetal adrenal gland and gonad. Recent Prog Horm Res 37: 41–103.
Voutilainen R, Ilvesmäki Miettinen P 1991 Low expression of 3β-hydroxy-5-ene steroid dehydrogenase gene in human fetal adrenals in vivo: adrenocorticotropin and protein kinase C-dependent regulation in adrenocortical cultures. J Clin Endocrinol Metab 72: 761–767.
Mesiano S, Coulter CL, Jaffe RB 1993 Localization of cytochrome P450 cholesterol side-chain cleavage, cytochrome P450 17α-hydroxylase/17,20-lyase, and 3β-hydroxysteroid dehydrogenase isomerase steroidogenic enzymes in human and rhesus monkey fetal adrenal glands: reappraisal of functional zonation. J Clin Endocrinol Metab 77: 1184–1189.
Endoh A, Kristiansen SB, Casson PR, Buster JE, Hornsby PJ 1996 The zona reticularis is the site of biosynthesis of dehydroepiandrosterone and dehydroepiandrosterone sulfate in the adult human adrenal cortex resulting from its low expression of 3 β-hydroxysteroid dehydrogenase. J Clin Endocrinol Metab 81: 3558–3565.
Gell JS, Atkins B, Margraf L, Mason JI, Sasano H, Rainey WE, Carr BR 1996 Adrenarche associated with decreased 3β-hydroxysteroid dehydrogenase expression in the adrenal reticularis. Endocr Res 22: 723–728.
Lebrethon MC, Jaillard C, Naville D, Bégeot M, Saez JM 1994 Effects of transforming growth factor-β1 on human adrenocortical fasciculata-reticularis cell differentiated functions. J Clin Endocrinol Metab 79: 1033–1039.
Miller WL, Auchus RJ, Geller DH 1997 The regulation of 17,20 lyase activity. Steroids 62: 133–142.
Morel Y, Mébarki F, Rhéaume E, Sanchez R, Forest MG, Simard J 1997 Structure-function relationships of 3 β-hydroxysteroid dehydrogenase: contribution made by the molecular genetics of 3 β-hydroxysteroid dehydrogenase deficiency. Steroids 62: 176–184.
Thomas JL, Berko EA, Faustino A, Myers RP, Strickler RC 1988 Human placental 3β-hydroxy-5-ene-steroid dehydrogenase and steroid 5→4-ene-isomerase: purification from microsomes, substrate kinetics, and inhibition by product steroids. J Steroid Biochem 31: 785–793.
Sauer LA, Chapman JC, Dauchy RT 1994 Topology of 3 β-hydroxy-5-ene-steroid dehydrogenase/δ5-δ4- isomerase in adrenal cortex mitochondria and microsomes. Endocrinology 134: 751–759.
Cherradi N, Defaye G, Chambaz EM 1994 Characterization of the 3β-hydroxysteroid dehydrogenase activity associated with bovine adrenocortical mitochondria. Endocrinology 134: 1358–1364.
Acknowledgements
The authors thank Dr. W. Miller for his methodologic suggestions. The collaboration of Sebastian Oknaian and Elina Feinstein is acknowledged.
Author information
Authors and Affiliations
Additional information
Supported by Agencia Nacional de Promocion Científica y Technologica of Argentina, Grant PID PMT-SID 0006, and Grant PMT-PICT 0028 and by the World Health Organization.
Rights and permissions
About this article
Cite this article
Dardis, A., Saraco, N., Rivarola, M. et al. Decrease in the Expression of the 3β-Hydroxysteroid Dehydrogenase Gene in Human Adrenal Tissue during Prepuberty and Early Puberty: Implications for the Mechanism of Adrenarche. Pediatr Res 45, 384–388 (1999). https://doi.org/10.1203/00006450-199903000-00016
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199903000-00016
This article is cited by
-
Adrenal changes associated with adrenarche
Reviews in Endocrine and Metabolic Disorders (2009)
-
Expression of the IGF and the aromatase/estrogen receptor systems in human adrenal tissues from early infancy to late puberty: Implications for the development of adrenarche
Reviews in Endocrine and Metabolic Disorders (2009)