Abstract
Intravascular and intraalveolar fibrin depositions in preterm infants with severe respiratory distress syndrome (RDS) have been attributed to activation of clotting. We questioned whether in the face of activated clotting, fibrinolysis is sufficient in these infants. We found, in infants with severe RDS within 6 to 12 h of birth, increased median thrombin-antithrombin III complex formation (11.1 versus1.3 ng/mL in the group with mild-to-moderate RDS, p < 0.001), indicating activation of clotting. Simultaneously, we found increased tissue-type plasminogen activator antigen (t-PA) release in plasma of these infants represented by increased median t-PA plasma concentrations (8.3 versus 2.5 ng/mL in the group with mild-to-moderate RDS, p < 0.01). This increased t-PA release was not accompanied with more plasminogen and antiplasmin consumption and with more fibrin and fibrinogen degradation than in the infants with mild-to-moderate RDS because plasma plasminogen and antiplasmin activity and total fibrin and fibrinogen degradation product concentrations were similar in both groups. We have found that activated clotting and t-PA plasma concentrations are positively correlated with arterial-to-alveolar oxygen tension ratio and ventilator efficiency index values. Plasminogen and antiplasmin activity, and total fibrin and fibrinogen degradation product concentrations were not correlated with these continuous measures of RDS severity. In neonatal RDS, clotting activity contributes to disease severity. Insufficient fibrinolysis likely facilitates the deleterious effects of activated clotting.
Similar content being viewed by others
Main
The presence of intravascular and intraalveolar fibrin depositions in the lungs of preterm infants with severe RDS(1,2) has been explained by activation of the clotting system(1,3–7). In these infants, activation of clotting is characterized by systemic generation of thrombin and consumption of AT III, the main inhibitor of thrombin(1,3–7). A further decrease of the preexisting low AT III plasma concentrations in preterm infants(8) results in insufficient neutralization of thrombin, leaving it capable of converting fibrinogen into fibrin. Both intravascular and intraalveolar fibrin depositions are thought to contribute to respiratory insufficiency, which might explain the association between systemic clotting activity and RDS severity(3). Intravascular fibrin depositions increase alveolar-capillary membrane permeability(9), thus contributing to formation of protein-rich pulmonary edema, whereas intraalveolar fibrin disturbs surfactant function(10,11).
Term and preterm newborn infants have poor defense to fibrin formation because of insufficient fibrinolytic activity(12–14). The key event of fibrinolysis is the conversion of plasminogen into plasmin, which is mediated by t-PA in the presence of fibrin. Plasmin, in turn, causes lysis of fibrin and fibrinogen resulting in formation of FDP(13,15,16). Plasmin activity is controlled directly by predominantly α2-antiplasmin(17), and indirectly by plasminogen activator inhibitors such as PAI-1(18). In healthy preterm infants, low plasminogen plasma concentrations and activity occur in the face of normal to high plasmin inhibitor concentrations and activity(8,12–14), thus creating a hypofibronolytic state. In preterm infants with severe RDS, increased plasma t-PA plasma concentrations have been found(4,5). However, fibrinolytic activity is probably insufficient for the amount of intravascular and intraalveolar deposition of fibrin in the lungs of these infants. We therefore studied simultaneously activation of fibrinolysis (t-PA, plasminogen and antiplasmin activity, total FDP) and clotting (platelet count, T-AT III complex) on the first and third day of life in preterm infants with clinical and radiologic signs of severe or mild-to-moderate RDS. We did not measure plasma concentrations of PAI-1.
METHODS
Patients. Twenty-six preterm infants, who were consecutively admitted to the neonatal intensive care unit of the Beatrix Childrens Hospital, University Hospital, Groningen, were studied. These infants met the following inclusion criteria for study enrollment: 1) no maternal infection, amnionitis, or prolonged rupture of membranes (>24 h before birth); 2) gestational age between 27 and 33 wk; 3) birth weight appropriate for gestational age; 4) no evidence of infection up to 3 d after study completion; and 5) no major congenital malformations. We realized that the clinical picture of transient tachypnea of the neonate might overlap with mild forms of neonatal RDS. We therefore considered infants who showed complete recovery from respiratory distress and associated chest x-ray abnormalities within 72 h of birth to have transient tachypnea of the neonate and excluded them from the study. All study infants showed clinical and radiographic signs of RDS. These infants were prospectively classified according to the clinical and roentgenologic criteria that were used in the Dutch multicenter study "Nedsurf" for surfactant replacement therapy. Eleven infants had severe RDS (severe RDS group), which was defined as extra oxygen requirement for adequate oxygenation, artificial ventilation dependency because of respiratory failure, and roentgenologic features on a chest x-ray consistent with a score of 3 or 4 according to Giedion et al.(19). Fifteen infants had mild-to-moderate RDS (mild-to-moderate RDS group), which was defined as extra oxygen requirement for adequate oxygenation, artificial ventilation or continuous positive airway pressure dependency because of respiratory failure, and roentgenologic features on a chest x-ray consistent with a score of 1 or 2 according to Giedion et al. The study was approved by the hospital ethical committee. Informed consent was obtained from the parents of all infants.
Several prenatal factors, such as premature labor; the syndrome of hemolysis, elevated liver enzymes, and low platelet count (HELLP) or toxicosis; mode of delivery; and drugs given to the mother, may influence the activation of clotting, fibrinolysis, and the platelet count. However, both RDS groups did not differ regarding these prenatal factors. The mothers of 3 infants in both the severe and the mild-to-moderate RDS group showed the syndrome of HELLP or toxicosis before delivery, which is sometimes accompanied by diminished platelet counts in the newborn infants(20). In this study, platelet counts of these infants could not be distinguished from those of the infants in both groups whose mothers did not show HELLP or toxicosis. The platelet counts of the 3 infants with mild-to-moderate RDS were higher than those of the 3 infants with severe RDS. The mothers of the infants in both RDS groups did not show other diseases that could influence the platelet count and the activation of clotting and fibrinolysis in their children. Dexamethasone was given to the mothers of 4 infants in the severe RDS group and to the mothers of 5 infants in the mild-to-moderate group more than 2 d before delivery. Dexamethasone is able to inhibit the fibrinolytic system(21). Despite maternal dexamethasone treatment activation of fibrinolysis was not different between these infants and the other infants in each group. No other specific drugs that could influence the activation of clotting and fibrinolysis, such as anticoagulants and cyclooxygenase inhibitors, were given to the mothers.
Postnatal characteristics of the severe and mild-to-moderate RDS groups are presented in Table 1. All infants of the severe RDS group had significantly lower 1- and 5-minute Apgar scores and lower arterial umbilical pH values than the infants of the mild-to-moderate RDS group, but showed no organ failure other than respiratory insufficiency from birth. Cranial ultrasonography was performed in all study infants by the attending radiologist, who was unaware of the severity of RDS. Intracranial hemorrhages, if present, were staged according to Levene et al.(22). We observed no grade IV intracranial hemorrhages in this study and only one grade III in an infant with severe RDS. Polycythemia (venous hematocrit > 0.65) was not observed in the infants of both groups throughout the study period.
Patient management. All study infants required respiratory support from birth to maintain the PaO2 between 7.5 and 10.0 kPa and the arterial PCO2 between 5.5 and 6.5 kPa. All infants with severe RDS and 10 infants with mild-to-moderate RDS received synchronized intermittent positive pressure ventilation (Babylog 8000, Dräger, Lübeck, Germany). Five infants with mild-to-moderate RDS required nasal continous positive airway pressure.
In this study, 2 infants with mild-to-moderate RDS received 50 mg/kg bovine surfactant (Alvofact, Boehringer, Ingelheim, Germany), whereas 9 infants with severe RDS were treated with 100 mg/kg surfactant endotracheally according to the criteria of the "Nedsurf study." Two infants with severe RDS did not receive surfactant because of suspicion of pulmonary hemorrhage.
Infants received transfusions of cryoglobulin-poor plasma or packed red blood cells with cryoglobulin-poor plasma to replace blood taken for routine laboratory tests. The severe RDS group received a slightly higher mean number of transfusions throughout the study period than the mild-to-moderate RDS group. The volume of these transfusions did not exceed 10% of the calculated blood volume in any 24-h period for each infant in the study. Cryoglobulin-poor plasma is stored frozen and contains only native inactive plasma proteins, whereas packed red blood cells contain only 10-20% plasma, including plasma proteins, which are slightly activated by the processing procedure(23). Therefore, we do not consider replacement transfusions to have influenced the findings in the infants of either study group.
Venous and arterial umbilical catheters and peripheral arterial catheters were only inserted or removed after approval of the attending neonatologist. The patency of arterial catheters was maintained by a continuous infusion of 0.9% NaCl solution containing 3U/mL heparin at a rate of 0.3-0.5 mL/h. Such low-dose heparin infusion does not influence clotting in preterm infants(24). All study infants had an arterial umbilical or peripheral arterial catheter, whereas 6 infants with severe RDS and 4 infants with mild-to-moderate RDS also had a venous umbilical catheter.
Study protocol. In all study infants, blood samples were taken from an indwelling arterial catheter. We attempted to minimize the influence of sampling on the studied parameters. Only blood samples collected from a free-flowing arterial catheter showing an undisturbed pressure curve were used for analysis. Umbilical catheters were known to be echographically patent. During sampling, the first 3 mL was not used for analysis in this study. All samples were collected in soft plastic tubes containing appropriate anticoagulant to prevent activation of blood before processing of the samples.
Blood samples were taken on the first day of life between 6 and 12 h of birth, and on the third day of life. The first sample was taken before any medical treatment, including replacement of surfactant and administration of blood products, had occurred. None of the infants required indomethacin for closure of an open ductus arteriosus or phototherapy for treatment of hyperbilirubinemia before completion of blood sampling on d 3. Each sample was obtained during routine blood sampling and used for measuring the platelet count and activation of clotting (T-AT III complex) and fibrinolysis (t-PA, plasminogen activity, antiplasma activity, total FDP). At each sampling, 0.3 mL of blood was anticoagulated with EDTA (0.01 M), for determination of the platelet count; 0.5 mL of blood was anticoagulated with citrate (0.3%), immediately centrifuged (1500 × g, 20 min) and the platelet-poor plasma stored at -20°C until determination of t-PA concentration, plasminogen and antiplasmin activity, and total FDP concentration. Another 0.5 mL of blood was collected in a tube containing hirudin-aprotinin and immediately centrifuged (1500 × g, 20 min). Afterward, the platelet-poor plasma was removed and stored at -20°C until the T-AT III concentration was determined.
In each study infant, the fraction of inspired oxygen (FiO2) was recorded, when a blood sample was taken. In study infants who were artificially ventilated, the PIP was recorded. In all study infants the PaO2/PAO2 ratio was calculated, and in the artificially ventilated infants the VEI was estimated. The PaO2/PAO2 ratio was calculated by dividing the PaO2 measured directly from an arterial blood sample by the PAO2 calculated using the following equation: PAO2 = FiO2 (PATM - PH2O) - PaCO2, where PATM is the atmospheric pressure (760 mm Hg), PH2O is the partial pressure of water vapor (47 mm Hg), and PaCO2 is the arterial carbon dioxide tension. The VEI was calculated according to Notter et al.(25) using the following equation: VEI = 3800 / (ΔP × F × PaCO2), where 3800 is a constant relating to CO2 production (mL·mm Hg·kg-1·min-1), ΔP is the PIP minus the positive end-expiratory pressure (cm H2O), and F is the ventilatory frequency. This index estimates the overall ventilation efficiency of mechanically ventilated infants accounting for the combined effect of different ventilatory pressures, rates, and PCO2 values. The VEI increases as lung function improves.
Assays. The platelet count was determined using a cell counter (Hemolog, Coulter Electronics, London, UK). Platelet counts < 50 × 109/L were verified by phase-contrast microscopy. The T-AT III complex concentration in plasma was measured by immobilization of the complex with europium-labeled monoclonal antibodies against the T-AT III complex (Est-1, American Diagnostica Inc., Greenwich, CT). The amount of bound europium label was counted by means of time-resolved fluorometry (Wallac, Turku, Finland). Reference values of the T-AT III complex concentration in plasma are 0-3.5 ng/mL. The t-PA concentration in plasma was determined similarly using europium-labeled monoclonal antibodies against t-PA (Esp-1, American Diagnostica Inc.). Reference values for plasma concentrations of t-PA are 0.5-5.0 ng/mL. The total FDP concentration was measured by means of ELISA (Fibrinostika TDP, Organon Teknika, Boxtel, The Netherlands). Reference values for total FDP plasma concentrations in adult plasma are 760 ± 640 ng/mL (mean ± SD). The activity of plasminogen and antiplasmin in plasma was determined by means of a colorimetric assay. For the plasminogen activity 25 µL of diluted plasma (10 µL of plasma and 390 µL of albumin) was incubated with 25 µL of plasmin (0.007 IU/mL; Sigma Chemical Co., St. Louis, MO) and 25 µL of fibrin monomers solution (1900 IU/mL; Chromogenix, Mölndal, Sweden). Then 25 µL of substrate S2403 (Chromogenix) and 25 µL of t-PA solution (Chromogenix) were added, and absorption was measured spectrophotometrically (Microplate Reader 3550 UV, Bio-Rad, Richmond, CA) at 405 nm every 5 min during 2 h at 37°C. After subtraction of absorption values measured in each plasma sample after addition of plasmin and saline instead of fibrin monomers and t-PA, the change in the remaining absorption values is a measure for plasminogen activity in each plasma sample. Plasminogen activity was expressed as international units per milliliter (reference values, 0.29-0.67 IU/mL) and calculated from a standard plasminogen dilution curve. For the determination of antiplasmin activity absorption was measured spectrophotometrically (Microplate Reader 3550 UV) at 405 nm every 5 min during 2 h at 37°C in each plasma sample after addition of plasmin, substrate S 2403, and saline. After subtraction of these absorption values from the absorption value measured in a plasmin-albumin solution, antiplasmin activity can be calculated. Antiplasmin activity was expressed as international units per milliliter (reference values 0.52-0.97 IU/mL).
Statistical analysis. Data are presented as mean ± SD or as median with 25th and 75th percentiles as appropriate. The χ2 test with Yate's correction for continuity was used for comparison of nominal data between the severe and mild-to-moderate RDS groups. Gestational age, birth weight, 1- and 5-minute Apgar scores, arterial umbilical pH values, and transfusion numbers in both groups were compared using the unpaired Student's t test.
For PIP values, platelet counts, and plasminogen and antiplasmin activity values, the unpaired Student's t test was performed to compare the values of these parameters between the two groups on d 1 (6 to 12 h) and d 3. A paired Student's t test was used to compare PIP values, platelet counts, and plasminogen and antiplasmin activity values on d 3 with the values of these parameters on d 1.
The Mann-Whitney U test and the Wilcoxon signed-rank test were used to determine specific differences between and within the two groups for the FiO2, PaO2/PAO2, and VEI values and the plasma T-AT III, t-PA, and total FDP concentrations.
Correlations were determined by calculating Spearman's rank correlation coefficient. Statistical significance was assumed when the p value was less than 0.05.
RESULTS
FiO2, PIP, VEI, PaO2/PAO2 ratio. On the first and third day of life median FiO2 and PaO2/PAO2 values of the infants with severe RDS were significantly higher than those of the infants with mild-to-moderate RDS (Table 2). Median FiO2 values did not change significantly between d 1 and d 3 in either study group. The median PaO2/PAO2 value of the severe RDS group was significantly higher on d 3 than on d 1 (Table 2).
Mean PIP values of the infants with severe RDS were higher than those of the artificially ventilated infants with mild-to-moderate RDS, achieving significance on d 3 (Table 2). The mean PIP value of the severe RDS group increased significantly between d 1 and d 3. The mild-to-moderate RDS group did not show significant changes of the PIP value (Table 2).
On the third day of life median VEI values were significantly lower in the severe RDS group than in the mild-to-moderate RDS group (Table 2). The median VEI value of the infants with severe RDS decreased significantly between d 1 and d 3, whereas that of the mild-to-moderate RDS group did not change significantly (Table 2).
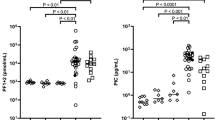
T-AT III complex concentration. T-AT III complex plasma concentrations were significantly higher in the severe RDS group than in the mild-to-moderate RDS group on d 1 (11.1 [6.6-24.5] ng/mL versus 1.3 [0.5-1.7] ng/mL [median, 25th-75th percentile]; p < 0.001) and d 3 (8.5 [3.8-32] ng/mL versus 1.3 [0.7-2.7] ng/mL, p < 0.01). Median T-AT III complex plasma concentrations of both RDS groups did not change significantly between d 1 and 3.
Platelet count. Mean platelet counts (± SD) of the infants with severe RDS were significantly lower than those of the infants with mild-to-moderate RDS on d 1 (156 ± 73 × 109/L versus 246 ± 69 × 109/L, p < 0.01) and d 3 (140 ± 80 × 109/L versus 242 ± 68 × 109/L, p < 0.01). The platelet count did not change throughout the study period in either RDS group.
t-PA concentration. On the first and third day of life t-PA plasma concentrations of the infants with severe RDS were significantly higher than those of the infants with mild-to-moderate RDS (Table 3). In neither RDS group were significant changes observed in the t-PA plasma concentration between d 1 and d 3.
Plasminogen and antiplasmin activity, total FDP concentration. Neither RDS study group differed with respect to their plasminogen activity, antiplasmin activity, and total FDP concentration on d 1 and d 3 (Table 3). These parameters did not change throughout the study period in either RDS group.
Correlations. Correlations were calculated between the values of the study parameters and the PaO2/PAO2 values for all study infants. Correlations between the study parameters and the VEI values were calculated for all artificially ventilated study infants. The PaO2/PAO2 ratio was negatively correlated with the plasma concentration of t-PA (ρ = -0.43; p < 0.01) (Fig. 1) and T-AT III complex (ρ = -0.54; p < 0.001) (Fig. 2) and positively correlated with the platelet count (ρ = 0.39; p < 0.05). The VEI was negatively correlated with the T-AT III complex concentration (ρ = -0.36, p < 0.05) and positively correlated with the platelet count (ρ = 0.36; p < 0.05), but was not correlated with the t-PA plasma concentration. The plasminogen activity, antiplasmin activity, and total FDP concentration did not show any significant correlation with the continuous measures of RDS severity.
DISCUSSION
Activation of clotting has been described in preterm infants with severe RDS(3–6). Simultaneously, increased plasma concentrations of t-PA has been found in these infants, indicating activation of the fibrinolytic system(4,5). However, because fibrin depositions have been found in the pulmonary microcirculation and in the small airways of preterm infants with severe RDS(1,2), fibrinolysis might be inadequate in these infants. In this study, we have found increased plasma concentrations of T-AT III complex and decreased platelet counts on the first and third day of life in 11 preterm infants with severe RDS (severe RDS group) indicating activation of clotting, which was almost absent in 15 preterm infants with mild-to-moderate RDS (mild-to-moderate RDS group). Simultaneously, we have observed significantly higher t-PA plasma concentrations in the severe RDS group than in the mild-to-moderate RDS group, whereas plasma plasminogen activity, antiplasmin activity, and total FDP concentrations were similar in both groups.
In the infants with severe RDS, we have found increased formation of thrombin indicated by increased T-AT III complex plasma concentrations. Thrombin is the central product of clotting. Normally, anticoagulant and fibrinolytic mechanisms limit clotting activation and localize thrombus formation. In severe RDS, thrombin inhibition is thought to be insufficient because of low AT III plasma concentrations(1,3,7), thus leaving it capable of activating platelets and converting fibrinogen into fibrin. The decreased platelet count in our severe RDS group is in agreement with previous findings(4–6,26) and likely reflects platelet activation and subsequent aggregation by thrombin. Intravascular and intraalveolar fibrin depositions, which have been found at autopsy in the lungs of preterm infants who died of severe RDS(1,2), may not only reflect unopposed thrombin formation but also insufficient fibrinolysis.
Normally, plasma t-PA and plasminogen concentrations of normal term and preterm newborn infants are lower than those of adults, whereas plasma concentrations of plasmin inhibitors are similar (α-2-antiplasmin, PAI-1) or even higher (α-2-macroglobulin, α-1-antitrypsin) than those of adults(8,12–14,18). Furthermore, plasma of normal term newborn infants shows slow plasmin generation when challenged by exogenous plasminogen activators(14). Reduced concentrations of t-PA and plasminogen accompanied with normal to increased concentrations of plasmin inhibitors creates a hypofibrinolytic state that limits the ability of the newborn infant to defend itself against intravascular fibrin clot formation(12). In this study, we have observed higher t-PA plasma concentrations in the infants with severe RDS than in the infants with mild-to-moderate RDS. This is in agreement with recent findings that have shown that both stressed preterm and term infants are able to increase t-PA levels(4,5,27). However, in our study, fibrinolytic activity did not differ between RDS groups. Plasma plasminogen activity, antiplasmin activity, and total FDP concentrations were similar in both groups. Actually, Table 3 shows that plasma plasminogen and antiplasmin activity values in the study infants were lower than in healthy preterm infants(8,12,14), whereas total FDP plasma concentrations were higher than those found in pooled plasma of healthy adults(28). These values indicate that fibrinolysis is similarly activated in both study groups, whereas one would expect more fibrinolytic activity in the infants with severe RDS than in those with mild-to-moderate RDS regarding the higher t-PA plasma concentrations. Insufficient t-PA activity probably explains this discrepancy between t-PA concentration and fibrinolytic activity in the severe RDS group. Normally, increase of t-PA plasma concentrations is accompanied by a simultaneous decrease of PAI activity(17). However, in stressed newborn infants it has been demonstrated that increase of t-PA is accompanied by increased PAI activity, which is thought to largely reverse t-PA activity(27). In addition to low plasminogen plasma concentrations, a disturbed balance between t-PA and PAI activity may result in impaired fibrinolytic activity in the severe RDS group, thus promoting intravascular and intraalveolar fibrin formation.
Increased t-PA plasma concentrations in plasma of the infants with severe RDS in our study seem to contrast with depressed t-PA concentrations in lung lavage material of similar infants in other studies(29,30). Because it is impossible from other studies and ours to establish the relative contribution of locally released versus plasma-derived t-PA to the t-PA concentration in the lungs of infants with severe RDS, we can only speculate about the aforementioned difference. The difference between plasma and lung lavage concentration of t-PA cannot be explained by differences in determination methods because both our determination method and the ELISA used in these other studies measure t-PA antigen in free and complexed form. However, one explanation might be that plasminogen is undetectable in lung lavage of preterm baboons with RDS(31), which might cause decreased local release of t-PA in the small airways of preterm infants with severe RDS. Furthermore, the characteristic protein-rich pulmonary edema in neonatal RDS likely dilutes intraalveolar factors such as t-PA, thus contributing at least in part to its lower concentrations in lung lavage fluid.
Products of clotting and fibrinolysis are considered to be important determinants of lung injury in the adult respiratory distress syndrome because they can damage pulmonary vascular endothelium directly or indirectly by neutrophil and platelet activation(9,32,33). Our study suggests, but does not prove, that clotting activation contributes to RDS severity because of the correlation between T-AT III plasma concentrations, platelet counts, and continuous measures of RDS severity (VEI, PaO2/PAO2). Furthermore, decreased platelet counts and increased plasma concentrations of T-AT III and t-PA were associated with lower Apgar scores and lower arterial umbilical pH values at birth and occurred simultaneously with increased FiO2 and PIP values in the infants with severe RDS. Hypoxemia and acidosis at birth are able to induce release of tissue factor and t-PA by activated endothelial cells(34–36), thus activating extrinsic clotting and fibrinolysis, respectively. Both hyperoxia and barotrauma cause lung tissue injury(37–39) and are associated with activation of factor XII and kallikrein in severe neonatal RDS(4,5,39). Activated factor XII and kallikrein contribute to clotting and fibrinolytic activity(40,41). We hypothesize that insufficient fibrinolytic activity will facilitate the deleterious effects of activated clotting. Low intraalveolar concentrations and activity of plasminogen and t-PA have been thought to favor intraalveolar formation of fibrin in preterm infants with RDS who develop bronchopulmonary dysplasia(29,31).
In conclusion, we have shown that activation of clotting and fibrinolysis occurs simultaneously in preterm infants with severe RDS during the first 3 d of life. It is suggested that fibrinolytic activity is insufficient in these infants because increased t-PA plasma concentrations are not accompanied by more plasminogen and antiplasmin consumption and total FDP formation than in the infants with mild-to-moderate RDS. Clotting activity likely contributes to RDS severity in the face of insufficient fibrinolysis.
Abbreviations
- RDS:
-
neonatal respiratory distress syndrome
- AT III:
-
antithrombin III
- T-AT III:
-
thrombin-antithrombin III complex
- t-PA:
-
tissue-type plasminogen activator antigen
- PAI-1:
-
plasminogen activator inhibitor-1
- total FDP:
-
total fibrin and fibrinogen degradation products
- FiO2:
-
fraction of inspired oxygen
- PIP:
-
peak inspiratory pressure
- PaO2/PAO2:
-
arterial-to-alveolar oxygen tension ratio
- VEI:
-
ventilator efficiency index
References
Peters M, Ten Cate JW, Breederveld C, De Leeuw R, Emeis J, Koppe J 1984 Low antithrombin III levels in neonates with idiopathic respiratory distress syndrome: poor prognosis. Pediatr Res 18: 273–276.
Stark CR, Abramson D, Erkan V 1968 Intravascular coagulation and hyaline membrane disease of the newborn. Lancet 2: 1180–1181.
Schmidt B, Vegh P, Weitz J, Johnston M, Caco C, Roberts R 1992 Thrombin/antithrombin III complex formation in the neonatal respiratory distress syndrome. Am Rev Respir Dis 145: 767–770.
Brus F, van Oeveren W, Okken A, Bambang Oetomo S 1994 Activation of the plasma clotting, fibrinolytic and kinin-kallikrein system in preterm infants with severe idiopathic respiratory distress syndrome. Pediatr Res 36: 647–653.
Brus F, van Oeveren W, Okken A, Bambang Oetomo S 1997 Disease severity is correlated with plasma clotting, fibrinolytic, and kinin-kallikrein activity in preterm infants wit respiratory distress syndrome. Pediatr Res 41: 120–127.
Brus F, van Oeveren W, Okken A, Bambang Oetomo S 1997 Increased release of elastase and thromboxane by a decreased number of circulating polymorphonuclear leukocytes and platelets in preterm infants with severe idiopathic respiratory distress syndrome. Pediatrics 99: 672–680.
Berg W, van den B, Breederveld C, Ten Cate JW, Peters M, Borm JJJ 1989 Low antithrombin III: accurate predictor of idiopathic respiratory distress syndrome in premature neonates. Eur J Pediatr 148: 455–458.
Peters M, Ten Cate JW, Jansen E, Breederveld C 1985 Coagulation and fibrinolytic factors in the first week of life in healthy infants. J Pediatr 106: 292–295.
Malik AB, Horgan MJ 1987 Mechanisms of thrombin-induced lung vascular injury and edema. Am Rev Respir Dis 136: 467–470.
Fuchimukai T, Fujiwara T, Takahashi A, Enhörning G 1987 Artificial pulmonary surfactant inhibited by proteins. J Appl Physiol 62: 429–437.
Ueda T, Ikegami M, Jobe A 1994 Surfactant subtypes. In vitro conversion, in vivo function, and effects of serum proteins. Am J Respir Crit Care Med 149: 1254–1259.
Corrigan JJ Jr 1992 Normal hemostasis in the fetus and newborn: coagulation. In: Polin RA, Fox WW (eds) Fetal and Neonatal Physiology, Vol 2. WB Saunders, Philadelphia, 1368–1371.
Corrigan JJ Jr 1988 Neonatal thrombosis and the thrombolytic system: pathophysiology and therapy. Am J Pediatr Hematol Oncol 10: 83–91.
Corrigan JJ Jr, Sleeth JJ, Jeter M, Lox CD 1989 Newborn's fibrinolytic mechanism: components and plasmin generation. Am J Hematol 32: 273–278.
Collen D 1985 Human tissue-type plasminogen activator. Circulation 72: 18–20.
Andrew M 1995 Developmental hemostasis: relevance to hemostatic problems during childhood. Semin Thromb Hemost 2: 341–356.
Aoki N, Saito H, Kamiya T, Koie K, Sakata Y, Kobakura M 1979 Congenital deficiency of α2-plasmin inhibitor associated with severe hemorrhagic tendency. J Clin Invest 63: 877–884.
Sprengers ED, Klutt C 1987 Plasminogen activator inhibitors. Blood 69: 381–387.
Giedion A, Haefliger H, Dangel P 1973 Acute pulmonary x-ray changes in hyaline membrane disease treated with artificial ventilation and positive end expiratory pressure. Pediatr Radiol 1: 145–152.
Brazy JE, Grimm JK, Little VA 1982 Neonatal manifestations of severe maternal hypertension occurring before the thirty-sixth week of pregnancy. J Pediatr 100: 265–271.
Jansen NJG, van Oeveren W, van Vliet M, Stoutenbeek CP, Eysman L, Wildevuur Ch RH 1991 The role of different types of corticosteroids in the inflammatory mediators in cardiopulmonary bypass. Eur J Cardiothorac Surg 5: 211–217.
Levene MI, Williams JL, Fawer CL 1985 Ultrasound of the Infant Brain. Clinics in Developmental Medicine, Vol 92. Blackwell Scientific Publications Ltd, Oxford
Gu YJ, Obster R, de Haan J, Gallandat Huet RCG, van Oeveren W 1992 Biocompatibility of leukocyte removal filters during bedside leukocyte filtration of red cell concentrates. Transfusion Sci 13: 467–472.
van Lingen RA, Hofhuis WDJ, Dekker I, Baerts W, Hählen K, Sauer PJJ 1992 The effect of heparin in arterial catheters on the coagulation in preterm infants. J Perinat Med 20: 39–46.
Notter RH, Egan EA, Kwong MS, Holm BA, Shapiro DL 1985 Lung surfactant replacement in premature lambs with extracted lipid from bovine lung lavage: effects of dose, dispersion technique and gestational age. Pediatr Res 19: 569–577.
Kohelet D, Perlman M, Hanna G, Ballin A 1990 Reduced platelet counts in neonatal respiratory distress syndrome. Biol Neonate 57: 334–342.
Corrigan JJ, Jeter MA 1992 Tissue-type plasminogen activator, plasminogen activator inhibitor, and histidine-rich glycoproteins in stressed human newborns. Pediatrics 89: 43–46.
Plötz FB, van Oeveren W, Barlett RH, Wildevuur CHRH 1993 Blood activation during neonatal extracorporeal life support (ECLS). J Thorac Cardiovasc Surg 105: 823–832.
Viscardi RM, Broderick K, Sun CJ, Yale-Loehr AJ, Hessamfar A, Taciak V, Burke KC, Koenig KB, Idell S 1992 Disordered pathways of fibrin turnover in lung lavage of premature infants with respiratory distress syndrome. Am Rev Respir Dis 146: 492–499.
Singhal KK, Parton LA 1996 Plasminogen activator activity in preterm infants with respiratory distress syndrome: relationship to the development of bronchopulmonary dysplasia. Pediatr Res 39: 299–235.
Idell S, Kumar A, Koenig KB, Coalson JJ 1994 Pathways of fibrin turnover in lavage of premature baboons with hyperoxic lung injury. Am J Respir Crit Care Med 149: 767–775.
Haynes JB, Hyers TM, Giclas PC, Franks JJ, Petty TL 1988 Elevated fibrin degradation products in ARDS. Am Rev Respir Dis 122: 841–847.
Sarnaik AP, Lieh-Lai M 1994 Adult respiratory distress syndrome in children. Pediatr Clin North Am 41: 337–364.
Nemerson Y 1992 The tissue factor pathway of blood coagulation. Semin Hematol 29: 170–176.
Manco-Johnson M 1992 Pathophysiology of neonatal disseminated intravascular coagulation and thrombosis. In: Polin RA, Fox WW (eds) Fetal and Neonatal Physiology, Vol 2. WB Saunders, Philadelphia, 1394–1399.
Collen D 1980 On the regulation and control of fibrinolysis. Thromb Haemost 43: 77–89.
Nilsson R, Grossmann G, Robertson B 1978 Lung surfactant and the pathogenesis of neonatal bronchiolar lesions induced by artificial ventilation. Pediatr Res 12: 249–255.
Saugstad OD 1990 Oxygen toxicity in the neonatal period. Acta Paediatr Scand 79: 881–892.
Saugstad OD, Buo L, Johansen HT, Roise O, Aasen AO 1992 Activation of the plasma kallikrein-kinin system in respiratory distress syndrome. Pediatr Res 32: 431–435.
Kaplan AP, Silverberg M 1987 The coagulation-kinin pathway of human plasma. Blood 70: 1–15.
Hathaway WE 1992 Normal hemostatic mechanisms. In: Polin RA, Fox WW (eds) Fetal Neonatal Physiology, Vol 2. WB Saunders, Philadelphia, 1365–1368.
Acknowledgements
The authors thank Johan Haan for his excellent technical support.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brus, F., Oetomo, S., Schieving, J. et al. Increased Tissue-Type Plasminogen Activator Antigen Release Is Not Accompanied by Increased Systemic Fibrinolytic Activity in Severe Neonatal Respiratory Distress Syndrome. Pediatr Res 45, 588–594 (1999). https://doi.org/10.1203/00006450-199904010-00020
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199904010-00020
This article is cited by
-
Activatie van plasma-eiwitten en bloedcellen bij het neonataal respiratoir distress-syndroom: pathogenetische en therapeutische aspecten
Tijdschrift voor kindergeneeskunde (2000)