Abstract
We hypothesized that antenatal exposure to glucocorticoids influences subsequent pulsatile cortisol (F) secretion in premature neonates. To test this hypothesis, blood was sampled for plasma F determination via indwelling arterial lines at 15-min intervals for 6 h in 26 clinically stable neonates whose gestational ages were 25-33 wk. Deconvolution analysis was used to characterize F secretion and elimination. Pulsatile F secretion was observed in all neonates. Deconvolution estimates in eight neonates exposed to antenatal glucocorticoids (ANG group) were compared with those of 18 neonates not or only remotely exposed to ANG (No/RG group). The median amplitude of the F secretory burst of the ANG group was significantly less than that of the No/RG group [4.3 nmol/Lv·min and 9.2 nmol/Lv·min, respectively; p = 0.026 (Lv is liter of F distribution volume)]. The number and duration of F secretory bursts was similar for both groups: 5 bursts per 6 h, and 23 versus 16 min. By univariate linear regression analysis, mean arterial blood pressure correlated positively with F secretory burst frequency and F production rate (p = 0.0035, r = 0.55 and p = 0.0067, r = 0.52, respectively). We propose that ANG treatment modulates the amplitude of pulsatile F secretion in premature neonates.
Similar content being viewed by others
Main
Antenatal corticosteroid therapy enhances fetal maturation and results in reductions in mortality, RDS, and intraventricular hemorrhage in neonates born between 24 and 34 wk GA (1,2). Hence, this therapy is advocated for women likely to deliver prematurely.
There are endocrine changes in premature neonates whose mothers have been treated with antenatal corticosteroids (3,4). Maternal betamethasone therapy is associated with suppression of neonatal plasma F levels, which return to normal by about 7 d (4–6). Most studies of plasma F in neonates exposed to ANG have involved either a single sample or a small number of samples.
Recent studies in neonates, involving repeated blood sampling at short intervals, have demonstrated that F is secreted in discrete pulses at about 80-min intervals (7,8). Adults and older children also secrete F in discrete pulses with approximately 9-30 secretory episodes in a 24-h period (9–12). These rapid pulsations of plasma F are termed ultradian rhythms, which are believed to be endowed by hypothalamic pituitary signaling. In addition, in adults and children a circadian rhythm is evident with maximal plasma F levels in the early morning and minimal levels in the evening (13,14). This circadian rhythm is not evident in the neonate and becomes established at about 3 months of age (15).
Pulsatile hormone data can be evaluated using deconvolution analysis, which is a mathematical technique to define pulsatile plasma hormone levels over time in relation to the underlying secretion and elimination characteristics of that hormone. To date, pulsatile plasma F secretion in neonates has been described in two studies using deconvolution analysis, with a total of 16 subjects of which only two neonates were less than 34 wk GA, and only one neonate was exposed to ANG (7,8). We prospectively sought to test the hypothesis that exposure to ANG influences the characteristics of pulsatile F secretion in neonates less than 34 wk GA. We specifically included a mixture of subjects who were exposed or not exposed to ANG.
METHODS
The study was performed in the Neonatal Intensive Care Unit at the Royal North Shore Hospital, Sydney, Australia. Twenty-six neonates whose GA ranged from 25 to 33 wk were studied. The ANG group consisted of eight neonates in whom the interval from ANG administration to time of study was less than 7 d. The remaining 18 neonates received no or remote glucocorticoids (ANG to study interval ≥7 d) and constituted the No/RG group. We chose the 7-d "cutoff" on the basis of the data of Ballard et al. (4). All but one of the mothers in the ANG group received two doses of betamethasone, 11.4 mg intramuscularly at an interval of 12 h, and the other mother received two doses of dexamethasone, 12 mg intramuscularly, also at an interval of 12 h. The ANG administration-time of study interval ranged from 2-5 d with a median of 4 d. The clinical characteristics of the subjects are given in Table 1. MABP and heart rate were recorded at 15-min intervals during the study, and the values were averaged to provide a single value for each neonate. BSA was estimated by the method of Haycock et al. (16).
Only clinically stable infants who had indwelling umbilical or peripheral arterial lines as part of their routine clinical care were studied. Each subject had a SNAP performed on the day of study to document the severity of illness at the time of study (17). The majority of infants (21 of 26) had only mild illness severity, with SNAP scores in the range 0-9. The remaining five infants had moderate illness severity, with SNAP scores in the range 10-19. Neonates were studied in the first 9 postnatal days, with one neonate being studied within 24 h of birth and an additional five neonates on the second day of life.
The principal diagnoses in the ANG group were RDS (n = 7) and pulmonary immaturity (n = 1). The principal diagnoses in the No/RG group were RDS (n = 15), pulmonary immaturity (n = 2), and transient tachypnea of the newborn (n = 1). Infants with RDS received intratracheal bovine surfactant before being studied. No subjects received postnatal glucocorticoids before being studied. Study subjects did not receive vasopressor or morphine infusions during the study. The number of infants who were intubated or extubated at the time of the study is given in Table 1. Informed parental consent was obtained, and the study was approved by the Medical Research Ethics Committee of the Royal North Shore Hospital.
Study protocol. Each neonate was studied on one occasion. Blood samples were obtained via indwelling arterial lines between 1200 and 1900 h for all subjects. Specimens were collected at 15-min intervals for a period of 6 hours, and a total of 2.6 mL of blood was taken from each subject. Before each blood sample, a volume of blood twice the dead space was withdrawn and all of this blood was returned immediately after sampling. The arterial line was flushed with 1 U/mL heparinized saline after each sample. No subject received a transfusion immediately before or during the 6-h study period. No subject was anemic as a consequence of this study. All samples were centrifuged at 12 500 rpm for 3 minutes and the heparinized plasma was stored at -20°C until analysis. During the 6-h blood sampling period some subjects required clinical interventions as a necessary part of routine clinical care, and the time of these interventions was prospectively recorded. For the purpose of this study, clinical interventions were defined as endotracheal suction (n = 10), endotracheal intubation (n = 1), and insertion of intravenous lines (n = 8). A total of 19 interventions were performed in 14 of the 26 subjects during the 6-h study period. The distribution of subjects who did and did not have interventions was similar in the ANG and No/RG groups (Table 1).
Assays. Plasma F concentrations were determined by RIA (Cortisol [125I] Radioimmunoassay Kit; Orion Diagnostica, Orion Corporation, Espoo, Finland). The RIA required 20 µL of plasma per F estimation, and the sensitivity of the assay was 6 nmol/L. All analyses from individual subjects were performed in a single run. The mean ± SD intraassay coefficient of variation was 2.9 ± 2.3%. The interassay coefficients of variation were 5.2-6.2%. In this assay, cross-reactivity values of the F antiserum were as follows: 11-deoxycortisol, 0.4%; corticosterone, 0.2%; aldosterone, <0.1%; cortisone, <0.1%; dehydroepiandrosterone sulfate, <0.1%; 17-OH-progesterone, <0.1%; and progesterone, <0.1%. Eighty-two percent of samples were analyzed in duplicate and 11% singly: there was insufficient sample for analysis in 7% of samples.
A single CBG level was estimated from pooled sera for each subject. The CBG assays were performed using a commercially available kit (CBG-RIA-100; IRE-Medgenix, Fleurus, Belgium). The sensitivity of the assay was 0.25 mg/L and the mean ± SD intraassay coefficient of variation was 2.67 ± 2.49%. A CBG level was determined in all eight of the ANG group subjects and 14 of the 18 No/RG group subjects; there was insufficient sample to estimate CBG in four subjects.
Deconvolution analysis. Deconvolution analysis is a mathematical technique used to analyze pulsatile hormone data. Details of this form of analysis have been reviewed (18–20). In summary, when plasma hormone concentrations fluctuate with time, deconvolution analysis considers this pulsatility in terms of its two components-a pulse of secretion and an elimination function. The combination of these two processes can be expressed as a convolution integral, which should approximate the original concentration versus time data Each secretory burst is characterized by its location in time, amplitude (maximal hormonal secretory rate achieved within the burst), half-duration (width of the burst at half-maximal amplitude), and mass secreted per burst (area of the calculated secretory burst). The interpulse interval is the time separating successive secretory burst centers. The frequency is the number of significant secretory peaks per sampling session (6 h in this study). The production rate is the product of mass per burst and number of bursts, plus any basal secretion. In this study, no estimation of basal F secretion was required to fit the data. The elimination function acts upon all hormone secreted and defines the plasma half-life. We assumed a monoexponential decay function for the metabolic clearance of F. Data are expressed in relation to subject-specific distribution volumes for F (Lv).
Statistical analysis. Some deconvolution parameters were not normally distributed, and the Mann-Whitney U test was used to compare the ANG and No/RG groups. To consider the possible effect of extreme prematurity on deconvolution parameters, we divided the No/RG group into two subgroups: ≤28 wk GA (n = 5) and >28 wk GA (n = 13) and compared the deconvolution parameters in the subgroups using the Mann-Whitney U test. For these analyses, a p value < 0.05 was considered statistically significant.
For all study infants, we used univariate linear regression analysis to examine relationships between plasma F, CBG concentrations, and the deconvolution parameters versus the clinical characteristics of the subjects: GA, birth weight, BSA, study day, SNAP score, and MABP. Because of the large number of comparisons, a protected p value < 0.01 was considered statistically significant for this analysis. All results are expressed as median and interquartile range unless stated otherwise.
RESULTS
There were no statistically significant differences in the clinical characteristics of the subjects between the ANG and No/RG groups (Table 1). The 6-h mean plasma F concentration (Table 2) was lower in the ANG group compared with the No/RG group, but this difference was not significant (117 nmol/L and 176 nmol/L, respectively; p = 0.13). The lowest and highest plasma F concentrations were 15 and 501 nmol/L, respectively. The median CBG concentration was 16 mg/L in the ANG group and 18 mg/L in the No/RG group (p = 0.076).
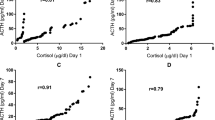
All 26 neonates secreted F in discrete pulses. Six neonates were studied in the first 2 postnatal days, including one neonate on the first day, demonstrating that pulsatile F secretion is established in premature neonates soon after birth. Figure 1 depicts the deconvolution analysis of a representative subject from each of the ANG and No/RG groups. The amplitude of the secretory burst appears lower in the ANG group subject compared with the No/RG group subject.
Results of deconvolution analysis of a representative subject from the No/RG group (left) and the ANG group (right). The gestational age of the No/RG subject was 29 wk and of the ANG subject, 27 wk. The upper subpanels show plasma F values at each sample time, with vertical error bars representing the intrasample SD. The continuous lines drawn through these points represent the reconvolved best fit of these data as predicted by deconvolution analysis. The lower subpanels depict the calculated F secretory rates, with each peak representing a discrete secretory burst. The amplitude is defined as the maximal F secretory rate achieved within the burst. The amplitude was lower in the ANG group subject than the No/RG group subject.
Results of deconvolution analysis for the two groups are summarized in Table 2. The median amplitude of the F secretory burst of the ANG group was less than half that of the No/RG group (4.3 nmol/Lv·min and 9.2 nmol/Lv·min respectively; p = 0.026). The number of secretory bursts was the same in both groups (5 per 6 h). The interpulse interval, F secretory burst half-duration, and mass of F selected per burst were similar in both groups. There was a trend for F production rate to be lower in the ANG group compared with the No/RG group, but this difference was not significant (438 nmol/Lv·6h and 749 nmol/Lv·6h, respectively; p = 0.085). The plasma F elimination half-life was slightly longer in the ANG group compared with the No/RG group, but this difference was not significant (73 min and 57 min, respectively; p = 0.085).
To investigate the possible effect of extreme prematurity on deconvolution parameters, we divided the No/RG group into two subgroups with five neonates having a GA ≤28 wk and 13 having a GA >28 wk. There was no statistically significant difference between mean plasma F, CBG, or any deconvolution parameter when the two GA subgroups were compared (data not shown).
Univariate linear regression analysis (Fig. 2) demonstrated that MABP positively correlated with F secretory burst frequency and F production rate (p = 0.0035, r = 0.55 and p = 0.0067, r = 0.52, respectively). No correlations with a p value < 0.01 were identified between 6 h mean plasma F, CBG, or the deconvolution parameters and any of the following: GA, birth weight, BSA, study day, and SNAP score.
Linear regression analysis of MABP and F secretory burst frequency and F production rate. Open circles denote ANG group subjects and filled circles, No/RG group subjects. MABP correlated positively with both F secretory burst frequency and F production rate (p = 0.0035, r = 0.55 and p = 0.0067, r = 0.52, respectively).
DISCUSSION
This study, using multiple blood sampling at short intervals and deconvolution analysis, had demonstrated that antenatal exposure to glucocorticoids results in a decrease in the amplitude of the F secretory burst in premature neonates. The study also confirms the findings in two previous studies that premature neonates secrete F in pulses at approximately 70-minute intervals and further characterizes pulsatile F secretion in the 25- to 33-wk GA range.
Pulsatile F secretion in premature neonates has been previously documented in only two small studies (7,8). The first was that of Metzger et al. (7), who studied five term and five premature neonates, none of whom was exposed to ANG. The second, by De Zegher et al. (8), included two term and four premature polycythemic neonates, one of whom had been exposed to ANG. In both studies, pulsatile F secretion was documented in all premature neonates, including the single infant exposed to ANG in the latter study. The present study is the first to study systematically the effect of ANG therapy on F production in premature neonates.
In our study the amplitude of the F secretory burst was significantly less in the ANG group than in the No/RG group, but the F secretory burst frequency was the same in both groups. We therefore propose that ANG exposure specifically modulates the amplitude of pulsatile F secretion in the premature neonate. This is in accord with data from studies of healthy adult males which have documented that the pulsatile secretion of ACTH and F is regulated predominantly by amplitude rather than frequency modulation (9,21). It is also in agreement with studies of the effect of postnatal dexamethasone therapy in infants with chronic lung disease on the hypothalamic-pituitary-adrenal axis, in which decreased basal ACTH levels and suppressed ACTH response to corticotropin-releasing hormone were found after dexamethasone therapy in infants born as early as 26 wk gestation (22–25).
Plasma CBG levels in the ANG group in our study were lower than in the No/RG group, but the difference was not significant. Kari et al. (26) demonstrated that dexamethasone therapy in premature neonates decreased the serum CBG concentrations and illustrated that CBG levels may be influenced by glucocorticoid treatment. Other studies have measured serum or plasma CBG levels in premature and term neonates and the values ranged from 13-26 mg/L, similar to those reported in our study (7,26,27).
The median value for plasma F for the No/RG group in our study was 176 nmol/L. This is similar to plasma F concentrations reported in premature infants in the first week of life in a number of studies (27–31). Many of the neonates in our study and these studies were on ventilators and hence the F levels seem inappropriately low given the clinical stresses they were enduring. In addition, healthy term neonates in the first week of life have plasma F levels similar to those in our study (32). This has led to the hypothesis that very premature neonates do not increase their F levels appropriately in response to clinical stresses and may manifest features of adrenal insufficiency (25–27,29,33).
Univariate linear regression analysis of our data including both groups demonstrated that MABP correlated with F production rate. There is some evidence that very premature neonates with hypotension respond to hydrocortisone administration with an increase in blood pressure, and some authors consider hypotension in neonates as a possible manifestation of hypocortisolism (34,35). In view of the association we have demonstrated between MABP and F production rate in our study, we hypothesize that it is those neonates with low F production rates who are likely to respond to exogenous hydrocortisone. However, determination of the efficacy of hydrocortisone in the treatment of hypotension in such premature neonates requires randomized, controlled trials.
We have demonstrated that the amplitude of the F secretory burst in neonates exposed to ANG is significantly less than that of nonexposed neonates and propose that ANG treatment modulates the amplitude, but not the frequency, of pulsatile F secretion in premature neonates. We also describe an association between MABP and F production rate and suggest that low F production rate may be a correlate of hypotension in neonates.
Abbreviations
- ANG:
-
antenatal glucocorticoids
- BSA:
-
body surface area
- CBG:
-
corticosteroid-binding globulin
- F:
-
cortisol
- GA:
-
gestational age
- MABP:
-
mean arterial blood pressure
- No/RG:
-
no or remote exposure to antenatal glucocorticoids
- RDS:
-
respiratory distress syndrome
- SNAP:
-
score for neonatal acute physiology
References
Crowley P 1995 Antenatal corticosteroid therapy: a meta-analysis of the randomized trials, 1972-1994. Am J Obstet Gynecol 173: 322–335
Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens Statement 1994 Feb 28-Mar 2; 12( 2): 1–24
Padbury JF, Ervin MG, Polk DH 1996 Extrapulmonary effects of antenatally administered steroids. J Pediatr 128: 167–172
Ballard PL, Gluckman PD, Liggins GC, Kaplan SK, Grumbach MM 1980 Steroid and growth hormone levels in premature infants after prenatal betametyhasone therapy to prevent respiratory distress syndrome. Pediatr Res 14: 122–127
Sippell WG, Bidlingmaier F, Knorr D 1980 Development of endogenous glucocorticoids, mineralocorticoids and progestins in the human fetal and perinatal period. Eur J Clin Pharmacol 18: 95–104
Collaborative Group on Antenatal Steroid Therapy 1981 Effect of antenatal dexamethasone administration on the prevention of respiratory distress syndrome. Am J Obstet Gynecol 141: 276–287
Metzger DL, Wright NM, Veldhuis JD, Rogol AD, Kerrigan JR 1993 Characterization of pulsatile secretion and clearance of plasma cortisol in premature and term neonates using deconvolution analysis. J Clin Endocrinol Metab 77: 458–463
De Zegher F, Vanhole C, Van Den Berghe G, Devlieger H, Eggermont E, Veldhuis JD 1994 Properties of thyroid-stimulating hormone and cortisol secretion by the human newborn on the day of birth. J Clin Endocrinol Metab 79: 576–581
Veldhuis JD, Iranmanesh A, Lizarralde G, Johnson ML 1989 Amplitude modulation of a burstlike mode of cortisol secretion subserves the circadian glucocorticoid rhythm. Am J Physiol 257: E6–E14
Kerrigan JR, Veldhuis JD, Leyo SA, Iranmanesh A, Rogol AD 1993 Estimation of daily cortisol production and clearance rates in normal pubertal males by deconvolution analysis. J Clin Endocrinol Metab 76: 1505–1510
Wallace WHB, Crowne EC, Shalet SM, Moore C, Gibson S, Littley MD, White A 1991 Episodic ACTH and cortisol secretion in normal children. Clin Endocrinol (Oxf) 34: 215–221
Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, Hellman L 1971 Twenty-four hour patterns of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol 33: 14–22
Esteban NV, Loughlin T, Yergey AL, Zawadzki JK, Booth JD, Winterer JC, Loriaux DL 1991 Daily cortisol production rate in man determined by stable isotope dilution/mass spectrometry. J Clin Endocrinol Metab 71: 39–45
Linder BL, Esteban NV, Yergey AL, Winterer JC, Loriaux DL, Cassorla F 1990 Cortisol production rate in childhood and adolescence. J Pediatr 117: 892–896
Vermes I, Dohanics J, Toth G, Pongracz J 1980 Maturation of the circadian rhythm of the adrenocortical functions in human neonates and infants. Horm Res 12: 237–244
Brion L, Fleischman AR, Schwartz GJ 1985 Evaluation of four length-weight formulas for estimating body surface area in newborn infants. J Pediatr 107: 801–803
Richardson DK, Gray JE, McCormick MC, Workman K, Goldmann DA 1993 Score for neonatal acute physiology: a physiologic severity index for neonatal intensive care. Pediatrics 91: 617–623
Veldhuis JD, Carlson ML, Johnson ML 1987 The pituitary gland secretes in bursts: appraising the nature of glandular secretory impulses by simultaneous multiple-parameter deconvolution of plasma hormone concentrations. Proc Natl Acad Sci U S A 84: 7686–7690
Veldhuis JD, Johnson ML 1990 A review and appraisal of deconvolution methods to evaluate in vivo neuroendocrine secretory events. J Neuroendocrinol 2: 755–771
Veldhuis JD, Johnson ML 1992 Deconvolution analysis of hormone data. Methods Enzymol 210: 539–575
Veldhuis JD, Iranmanesh A, Johnson ML, Lizarralde G 1990 Amplitude, but not frequency, modulation of adrenocorticotropin secretory bursts give rise to the nyctohemeral rhythm of the corticotropic axis in man. J Clin Endocrinol Metab 71: 452–463
Alkalay AL, Pomerance JJ, Puri AR, Lin BJC, Vinstein AL, Neufeld ND, Klein AH 1990 Hypothalamic-pituitary-adrenal axis function in very low birth weight infants treated with dexamethasone. Pediatrics 86: 204–210
Wilson DM, Baldwin RB, Ariagno RL 1988 A randomized, placebo-controlled trial of effects of dexamethasone on hypothalamic-pituitary-adrenal axis in preterm infants. J Pediatr 113: 764–768
Rizvi ZB, Aniol HS, Myers TF, Zeller WP, Fisher SG, Anderson CL 1992 Effects of dexamethasone on the hypothalamic-pituitary-adrenal axis in preterm infants. J Pediatr 120: 961–965
Hanna CE, Keith LD, Colasurdo MA, Buffkin DC, Laird MR, Mandel SH, Cook DM, LaFranchi SH, Reynolds JW 1993 Hypothalamic pituitary adrenal function in the extremely low birth weight infant. J Clin Endocrinol Metab 76: 384–387
Kari MA, Raivio KO, Stenman U-H, Voutilainen R 1996 Serum cortisol, dehydroepiandrosterone sulfate, and steroid-binding globulins in preterm neonates: effect of gestational age and dexamethasone therapy. Pediatr Res 40: 319–324
Scott SM, Watterberg KL 1995 Effect of gestational age, postnatal age, and illness on plasma cortisol concentrations in premature infants. Pediatr Res 37: 112–116
Lee MM, Rajagopalan L, Berg GJ, Moshang T 1989 Serum adrenal steroid concentrations in premature infants. J Clin Endocrinol Metab 69: 1133–1136
Hingre RV, Gross SJ, Hingre KS, Mayes DM, Richman RA 1994 Adrenal steroidogenesis in very low birth weight preterm infants. J Clin Endocrinol Metab 78: 266–270
Wittekind CA, Arnold JD, Leslie GI, Luttrell B, Jones MP 1993 Longitudinal study of plasma ACTH and cortisol in very low birth weight infants in the first 8 wk of life. Early Hum Dev 33: 191–200
Noguchi A, Reynolds JW 1978 Serum cortisol and dehydroepiandrosterone sulfate responses to adrenocorticotropin stimulation in premature infants. Pediatr Res 12: 1057–1061
Wiener D, Smith J, Dahlem S, Berg G, Moshang T 1987 Serum adrenal steroid levels in healthy full-term 3-day-old infants. J Pediatr 110: 122–124
Korte C, Styne D, Merritt TA, Mayes DM, Wertz A, Helbock HJ 1996 Adrenocortical function in the very low birth weight infant: improved testing sensitivity and association with neonatal outcome. J Pediatr 128: 257–263
Helbock HJ, Insoft RM, Conte FA 1993 Glucocorticoid-responsive hypotension in extremely low birth weight newborns. Pediatrics 92: 715–717
Colasurdo MA, Hanna CE, Gilhooly JT, Reynolds JW 1989 Hydrocortisone replacement in extremely premature infants with cortisol insufficiency. Clin Res 37: 180A( abstr)
Acknowledgements
The authors thank the nursing staff of the Newborn Intensive Care Unit, Royal North Shore Hospital and Dr. Barbara Blades, Endocrine Laboratory, New Children's Hospital, Westmead, for their expert assistance. We also acknowledge the excellent secretarial support provided by June Drysdale. Dr. Arnold, Dr. Leslie, and Professor Silink are affiliated with the Department of Paediatrics and Child Health, University of Sydney.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Arnold, J., Bonacruz, G., Leslie, G. et al. Antenatal Glucocorticoids Modulate the Amplitude of Pulsatile Cortisol Secretion in Premature Neonates. Pediatr Res 44, 876–881 (1998). https://doi.org/10.1203/00006450-199812000-00009
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199812000-00009
This article is cited by
-
Glucocorticoids and fetal programming part 1: outcomes
Nature Reviews Endocrinology (2014)
-
Serum cortisol values, superior vena cava flow and illness severity scores in very low birth weight infants
Journal of Perinatology (2010)
-
Relative adrenal insufficiency in the preterm and term infant
Journal of Perinatology (2009)