Abstract
This study was designed to evaluate upper airway protective mechanisms in response to pharyngeal fluid stimulation in healthy term and preterm infants at term equivalent age. Five term and seven preterm infants were studied and the following recorded: sleep state, cardiorespiratory function, and swallowing. Infusions of 0.9% saline and sterile water of volumes of 0.04, 0.2, and 0.35 mL were made during active (AS) and quiet sleep (QS). The effect of these variables on apnea (≥2 and ≥5 s), swallowing, and arousal was examined. After pharyngeal infusion, apnea of ≥2 and ≥5 s did not change from spontaneous rate for both term and preterm infants. The most common response to pharyngeal infusion was swallowing. In AS, swallowing occurred after 65 and 73% and in QS after and 64% of infusions in term and preterm infants, respectively. Swallowing was volume-related and occurred significantly more often in term infants after larger infusions of 0.35 and 0.2 mL (83 and 67%) compared with the 0.04 mL (19%) and after 0.2 mL compared with 0.04 mL for preterm infants (94 and 44%). At 0.2 mL, this was significantly higher in preterm compared with term infants (p < 0.01) and was the only significant difference between these infants. In response to pharyngeal fluid stimulation, airway defense in both full-term and preterm infants is maintained primarily by swallowing with no evidence of apnea.
Similar content being viewed by others
Main
Recent work established that pharyngeal fluid infusion during sleep in the newborn piglet evoked swallowing, arousal, and occasionally expiratory reflex, and apnea did not occur (1). In contrast, comparable studies undertaken by other research groups in older infants (2), term (3), and preterm infants (3–6) reported that pharyngeal fluid infusion commonly evoked brief central apnea and occasionally prolonged apnea. The authors of these human studies concluded that prolonged apnea is characteristic of an exaggerated upper airway protective response (6). Furthermore, they proposed LCR stimulation in the generation of these responses (4,5). This is of particular relevance for SIDS, because the LCR is age-related and most severe in the young infant (7), and this reflex has been proposed as a possible mechanism of sudden and unexplained infant death (8,9).
Why the LCR is not evoked after pharyngeal fluid infusion in the sleeping and intact newborn piglet, but in comparable circumstances is evoked in the sleeping human infant is not clear, but may be attributed to several factors. The responses may reflect differences in species, newborn piglets compared with human neonates. Alternatively, the findings may differ because of confounding factors. These include the uncertainty of whether the term infants studied were selected from a healthy population, because three of the infants were siblings of SIDS infants, one was a half sib of a SIDS infant and the remaining infant was in the hospital recovering from pneumonia (3). Second, the different responses may also reflect the variable maturation of the preterm infants, because the responses were elicited at different postmentrual ages (27-36 wk) (3–6). In addition, sleep state that alters LCR-mediated responses (10–12) was not defined.
The aim of this study was to further investigate the mechanisms that protect the upper airway in human infants. The study compared responses evoked after infusion of small boluses of saline and water into the pharynx of healthy, sleeping term infants and preterm infants at term equivalent age. The hypotheses were that healthy, newborn infants, like piglets, would protect the airway by swallowing and arousal in response to fluid in the pharynx, whereas airway protection in preterm infants at an equivalent postmenstrual age would be depressed and resemble a LCR response.
METHODS
Subjects and selection. Five healthy term and seven preterm infants at term equivalent age (37-42 wk) were successfully studied at King George V Hospital between May and August 1995. The experimental conditions were kept constant for both the term and preterm infants. The experimental protocol was similar to that which has been previously described in term and preterm infants (3) and healthy, sleeping piglets (1). The ethics committee of Royal Prince Alfred Hospital approved the experimental protocol. Written informed consent was obtained from the parents of each infant who participated in the study.
The term infants were selected from the maternity ward at King George V Hospital. Selection for entry into the study required that the infants were born at a gestational age of 37-42 wk (as routinely determined by maternal dates in conjunction with ultrasound examination before 20 wk of gestation). In addition, entry criteria required that the infants were not treated for jaundice and were aged 3-5 d at the time of study and of a weight and length appropriate for gestational age according to Australian growth charts (13). Entry also required that the infants were born by normal vaginal delivery to mothers who had no complications of pregnancy and had not received epidural analgesic drugs via the intramuscular or epidural routes during labor.
The seven preterm infants, who had been admitted to the neonatal intensive care unit at King George V Hospital after their very preterm birth, were successfully studied at a postmenstrual age equivalent to term gestation (37-42 wk). Inclusion criteria required that the infants were born between 24 and 32 wk of gestation, at term equivalent age (37-42-wk postmenstrual age) at the time of study, and found to have a normal head ultrasound at 1 wk and 1 mo of age. A normal head ultrasound was defined as no evidence of grades III or IV intraventricular hemorrhage or periventricular leukomalacia and is the best predictor of normal outcome at long-term follow-up (14). Two of the preterm infants had been diagnosed at 28 postnatal days as having chronic lung disease, determined by serial x-rays and oxygen dependency, and required low flow nasal oxygen at the time of study.
At the time of study none of the infants had significant apnea of prematurity or were being treated for apnea. In addition none of the infants were symptomatic for GER. Apgar scores at 1 and 5 min were analyzed, and a significant difference was found between the term and preterm infants (p < 0.01). Apgar scores (median and range) at 1 min was 9 (8,9) and at 5 min was 10 (9,10) for term infants and 4 (4,8) and 8 (6,9) for preterm infants, respectively.
Recordings. For each recording session, the infants were instrumented and slept supine with their head in a position of lateral recumbency in the same quiet, warmed room after their morning milk feed.
Polygraphic recording of sleep state. Sleep state was determined by examination of multichannel pen and computer on-line recordings, and constant documentation of behavior. To determine sleep state, electrodes for recording EEG, in addition to piezoelectric crystals for recording electro-occulogram, and body movement were used. Sleep was staged in the 70-s epoch immediately before each infusion of fluid into the pharynx, according to the criteria of Anders et al. (15). Sleep was classified as being AS, QS, or indeterminate. During sleep, behavioral observations such as coughs, sneezes, and shudders were also noted on the paper traces and entered directly into the computer as a comment.
Cardiorespiratory measurements included nasal airflow, recorded using a Premie Airflow Sensor (Edentec Corp., Eden Prairie, MN) placed under the external nares of the infant, and chest wall movements from a mercury-in-rubber strain gauge (Pneumo-trace) taped across the baby's abdomen and connected to a plethysmograph (model 270, Parks Electronics Laboratory, Beaverton, OR). Heart rate and Sao2, were detected using a sensor wrapped around the foot of the infant and connected to a pulse oximeter (Nellcor II N-25 Neonatal/Adult O2 transducer, Hayward, CA) sampling at 3 Hz with an averaging time of 3 s.
Respiratory rate was calculated by counting the number of completed breaths resulting in airflow in the 10 s immediately before and after each infusion of fluid into the pharynx and was expressed as a percent change in breaths/min. Sao2 (%) and heart rate (beats/min) were averaged in the 10-s period before each infusion and were compared with the lowest values reached within the 30 s immediately after each infusion.
Apnea. Apnea was defined as a cessation of nasal airflow over one of two minimum time periods for comparison with previously published data, the first ≥2 s (3) and second ≥5 s (1). A distinction was made between three types of apnea, central, obstructive, or mixed. Central apnea was defined as a cessation of both breathing movements and nasal airflow, obstructive apnea as a cessation of nasal airflow with continued breathing movements, and mixed if there was a combination of central apnea with at least three obstructed breaths in the one apneic event (16). The end of the apneic episode was defined as the resumption of two or more completed breaths on the nasal flow trace within 3 s.
Airway protective responses. Airway protective responses, namely swallowing, arousal, and expiratory reflexes (cough or sneeze), and apnea were determined from the pen and computer on-line recordings in addition to the behavioral observations. Swallowing and esophageal motility were monitored using a custom made Mikro-Tip catheter containing four solid-state microtransducers (model SSD-578 Millar Instruments Inc., Houston, TX) in conjunction with a Millar catheter pressure transducer system (4x model TC-510 Transducer Control Units). The distal pressure transducer was set in the tip of the catheter, with the subsequent transducers positioned at distances of 9.0, 12.0, and 15.5 cm from the distal transducer. The catheter (size 6 French gauge) was inserted nasogastrically such that the proximal pressure transducer was positioned in the pharynx. Correct positioning of the pharyngeal transducer was achieved by the identification of sharp onset pressure rises characteristic of pharyngeal swallows (3,4) on a 12-channel pen recorder (model 78D; Grass Instruments Co., Quincy, MA). Once the proximal transducer was positioned in the pharynx as described, the subsequent transducers were situated in the upper esophagus, lower esophagus, and stomach. This arrangement of pressure transducers facilitated the clear monitoring of swallowing and other paryngeal and esophageal body movements, as well as allowing changes in intragastric pressure to be detected.
The occurrence, rate, and latency to the first swallow were analyzed. The latency to swallow was defined as the time from the infusion of the fluid bolus into the pharynx to the beginning of the pharyngeal contraction. Arousal was defined by electrophysiologic and behavioral criteria, in that the EEG trace showed low voltage, fast waves, the eyes were open, and body movement occurred (observed visually and seen as a movement artifact on the chart recording) with or without an expiratory reflex. Behavioral arousal can be clearly defined and was selected as the end point in this study as it is very difficult to determine a universally accepted definition of polygraphic arousal. Expiratory reflexes were identified aurally and confirmed by a characteristic esophageal pressure trace (3).
In addition to the 12-channel pen recordings, all infusion studies were recorded and stored using a Macintosh computer data acquisition system (model MP 100 data acquisition system, Biopac Systems Inc., Goleta, CA) and software (Acq-Knowledge 881 version 3.1.2). Traces were rejected if the results were obscured by a movement artifact.
Stimuli. During AS and QS, fluid boluses (0.9% saline and sterile water), were administered into the pharynx via a 5-French gauge feeding tube positioned through the nose and taped to the infant's face to ensure the fluid boluses were administered at skin temperature (3,4). The infusion catheter was positioned such that the opening was in the pharynx in line with the proximal pressure transducer on the manometric catheter. Correct positioning of the pharyngeal transducer at the junction of the oropharynx and hypopharynx was confirmed by the identification of sharp onset pressure rises characteristic of pharyngeal swallows (3,4). At least 2 min elapsed between the delivery of each bolus. The type of fluid and the volume of each bolus was administered in random order both within and between experiments. The experiments were halted when the protocol was completed or the infant aroused and would not return to sleep.
Statistical analysis. Control periods were defined as 1-min epochs beginning 70 s before each infusion of the fluid. To meet the criteria for receiving a stimulus, the remaining 10 s immediately preceding the infusion was a period in which the infant was lying quietly without any apnea or swallows. The responses were determined in the control periods and the 10-s period immediately after infusion and expressed as either an occurrence (categorical data), or rate/min (continuous data).
Results for the categorical data are expressed as a percentage of the total number of infusions performed. All results for continuous data are expressed as the mean and (SEM), because these parameters were the most appropriate for describing these data.
The responses evoked after each infusion, namely swallowing, arousal expiratory reflexes, apnea, and change in respiratory rate, heart rate, and Sao2 were compared with the control periods. The effect of gestational age (term versus preterm), sleep state (AS, QS), infusate (saline, water), and volume of infusate (high 0.35 mL, medium 0.2 mL, and 0.04 mL) on each of the responses was examined. The largest of the volumes was administered in the term infants only.
The χ2 test was used to examine the effect of gestational age, sleep state, infusate, and volume of infusate on the categorical data, occurrence of swallowing, arousal, expiratory reflexes, and apnea. The Kruskal Wallis one-way ANOVA was used to examine the effect of age, sleep state, infusate, and volume on the continuous variables, that is rate of and latency to swallowing and change in respiratory rate. If the Kruskal Wallis test indicated significant differences, the Mann-Whitney U test was used to determine where the differences were.
Physiologic GER is an event that occurs in every infant. If GER evokes a life-threatening response, the response it evokes must be an abnormal one, for clearly, the particular episode of GER which leads to this response is only one of a large number which would have occurred in any particular infant. This study was designed therefore to introduce a large number of stimuli (simulated pharyngeal GER) and examine the variability in response to each stimulus in the infant groups. Based on analysis of a previous study (1), power analysis indicated significant differences in response to simulated pharyngeal reflux would be detected with a sample size of between 20 and 45 with a set at 0.05 and b set at 0.2. In total, there were 229 infusions, 105 were made in the term infants and 124 in the preterm infants.
Multiple comparisons were chosen as the method of analysis because each infusion and the responses which occurred were independent of every other infusion (that is there was no habituation to the test fluids within each infant). All statistical analyses were completed using the Statistical Package for Social Sciences (SPSS/PC+ version 4.0) program (17). Probability values of p < 0.01 were considered significant.
RESULTS
Infants. Infusion studies were undertaken and completed in five healthy, term infants and seven preterm infants at term equivalent age. The gestational, postnatal, and postmenstrual ages (X¯ ± SD) of the term infants were 39.2 ± 0.8 wk, 4.0 ± 1.0 d, and 39.8 ± 0.8 wk and for the preterm infants 26.6 ± 1.7 wk, 91.4 ± 14.8 d, and 39.6 ± wk, respectively. Although there was a significant difference between gestational and postnatal ages of the infants (p < 0.01), there was no significant difference in the postmenstrual age at the time of study between the term and preterm infants. During the 12 recording sessions, a total of 229 pharyngeal infusions were made, 105 in term infants of which 67 were in AS and 38 in QS, and 124 infusions in the preterm infants of which 80 were in AS and 44 in QS.
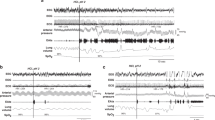
Responses. After each infusion, the onset of the responses usually occurred within 10 s. The swallow response between subjects was reproducible, and the results are in Tables 1–3. An example of the physiologic on-line recording obtained during the infusion protocol is illustrated in Figure 1.
An example of the computer-generated on-line recordings. The unboxed numbers on the x axis represent time (in seconds) from the beginning of each record. The solid arrowheads at the top of the graph depict behavioral observations/comments, the solid vertical lines indicate the point of comment entry. The period before infusion of 0.35 mL of saline shows regular breathing, behavioral quiescence, and trace alternate pattern on the EEG, typical of QS. After infusion, one swallow occurred within 3.4 s, a slight pause in breathing and a small head movement associated with this occurred, and the infant remained asleep.
Apnea (≥2 s). During control periods, sleep state had a significant impact on occurrence of apnea. Any apnea lasting 2 s or more occurred during 48% of control periods in AS and 17% in QS in term infants, and 58 and 9%, respectively, for preterm infants (p < 0.01) (Fig. 2, A and B). In AS, for both term and preterm infants, there was no change in the occurrence of apnea, after pharyngeal infusion. In term infants, apnea occurred in 48% of control periods, and after 17% of saline and 27% of water infusions. Similarly, in the preterm infants apnea occurrence did not change from spontaneous values, apnea occurred in 58% of control periods, and after 31% of saline and 34% of water infusions (Fig. 2A). In QS, pharyngeal infusion did not affect apnea occurrence in term or preterm infants. In term infants, apnea occurred in 17% of control periods, and after 25% of saline and 29% of water infusions, the results for preterm infants were 9, 19, and 30%, respectively (Fig. 2B).
The occurrence of apnea ≥2 s (% of the total infusions). (A) In AS, for both term and preterm infants, there was no change in the occurrence of apnea after pharyngeal infusion. In term infants, apnea occurred in 48% of control periods, and after 17% of saline and 27% of water infusions. Similarly, in the preterm infants apnea occurrence did not change from spontaneous values, apnea occurred in 58% of control periods, and after 31% of saline and 34% of water infusions. The occurrence of brief central apnea is sleep state-related, and occurs significantly more in AS compared with QS. Any apnea lasting 2 s or more occurred in 48% of control periods in AS and 17% in QS in term infants, and 58 and 9%, respectively, for preterm infants (*p < 0.01). (B) In QS, the occurrence of apnea after pharyngeal infusion was not significantly different from control values in either the term or preterm infants. Apnea occurred in 17% of control periods and after 25% of saline and 29% of water infusions. Results for preterm infants were 9, 19, and 30%, respectively.
When the duration of apneic episodes was examined (X¯ ± SEM), term infants had spontaneous episodes lasting 7.4 (0.9 s) and the preterm infants were 5.3 (0.5 s). The apnea duration did not change, after pharyngeal fluid infusion, and was 3.7 (0.4 s) and 4.4 (0.5 s), respectively.
Apnea (≥5 s). Neither sleep state nor gestational age affected the occurrence of apnea lasting 5 s or more. Overall, term infants had spontaneous apneic episodes >5 s, in 16% of the control periods and after 4% of infusions, whereas preterm infant results were 8 and 8%, respectively. Collectively this amounted to spontaneous apnea in 11% of control periods and after 6% of infusions, with a duration of 6.6 (0.3 s) and 7.6 (0.7 s), respectively.
Hierarchy of responses. In AS, swallowing was the most common response after infusion of fluid into the pharynx. Other responses in decreasing incidence were arousal and an expiratory reflex. During AS in term infants, swallowing, arousal, and an expiratory reflex occurred after 65, 16, and 6% of the total infusions performed; in preterm infants the responses were 73, 14, and 6%, respectively. In QS, swallowing occurred after 40% of infusions in term and 64% of infusions in preterm infants; however, arousal or expiratory reflexes were never evoked. For the arousal response in both term and preterm infants, this represented a significant difference between AS and QS (p < 0.01).
Swallowing. Sleep state significantly affected both the occurrence and frequency of spontaneous (control) swallowing in both term and preterm infants. In AS, spontaneous swallowing occurred significantly more often in control periods compared with QS (p < 0.01) (Table 1). Similarly, swallowing rate (swallows/min) was significantly greater in AS compared with QS (p < 0.01) (Table 1). In contrast, sleep state had no effect on the occurrence or frequency of swallowing after fluid infusion.
In AS the occurrence of swallowing did not significantly change from control values after infusion of either saline or water for both term and preterm infants (Table 1). In QS, the trend was similar in term infants, there was no significant change from control values after saline and water infusions. Preterm infants however significantly increased swallow occurrence from control values after infusions of saline and water (p < 0.01) (Table 1).
Infusion of the larger bolus volumes (high and medium) significantly increased swallow occurrence compared with low volumes (p < 0.01) (Table 2). Preterm infants exhibited the same response, in that medium volumes evoked significantly more swallows when compared with low volumes (p < 0.01) (Table 2). When the term and preterm infants were compared across similar volumes, the preterm infants swallowed significantly more after medium volumes only (p < 0.01). For all of the outcome variables measured, this was the only one that showed a significant difference between the term and preterm infants.
The nature of the fluid infused significantly altered the swallowing rate (swallows/min). For the term infants in AS, control frequency of swallowing did not increase significantly after infusions of saline, but did increase significantly after administration of water when compared with control and saline infusions (p < 0.01) (Table 1). In QS, the swallowing rate increased after infusions of water compared with that of control (p < 0.01). Results for saline were intermediate, but did not differ significantly from either control or water (Table 1). In preterm infants in AS, infusion of either fluid significantly increased the rate of swallowing from control values (p < 0.01) (Table 1). In addition, the swallowing rate was significantly increased after water compared with saline infusions (p < 0.01). In QS, infusion of both saline and water increased the rate of swallowing from control (p < 0.01), however, there was no significant difference between the number of swallows evoked after saline and water infusions (Table 1).
The volume of the fluid bolus significantly affected swallowing rate in both term and preterm infants in response to both saline and water infusions. In term infants, infusions of high and medium volumes of saline evoked significantly more swallows compared with low volumes (p < 0.01) (Table 2). The trend was the same after water infusions in term infants (p < 0.01) (Table 2). Administration of medium volume infusions evoked significantly more swallows than low volume infusions in preterm infants for both saline and water (p < 0.01) (Table 2).
The latency to the first swallow was not affected by the type of the infusate in both groups of infants (Table 3). The latency to the first swallow was significantly shorter after infusions of high and medium volumes compared with low volume infusions in term infants only (p < 0.01) (Table 3). Volume had no effect on the latency to the first swallow in preterm infants (Table 3).
Cardiorespiratory responses. Pharyngeal infusion evoked small, although not statistically significant changes in the respiratory rate. On average, the respiratory rate decreased 11.6% (3) in term infants and 11.6% (2.9) in preterm infants. The fluid infused had no significant effect on the change in respiratory rate in either group. Furthermore, bolus volume did not affect the respiratory rate in term infants, however, in preterm infants medium volumes decreased the respiratory rate significantly more 23.5 (3.9) than small volumes 0.6 (3.7 breaths/min) (p < 0.01).
After pharyngeal infusion of fluid, Sao2 and heart rate were maintained at all times during AS and QS in both groups of infants. The overall change in mean Sao2 in the term infants amounted to a decrease of 0.5% (0.1), and in the preterm infants not receiving oxygen 1.0% (0.2). There were small reductions in heart rate after infusion. Although not statistically significant, heart rate decreased on average, 8.8 (1.4 beats/min) in term infants and 13.5 (1.7 beats/min) in preterm infants. Neither the fluid infused or the volume of the fluid had any effect on the change in Sao2 or heart rate.
DISCUSSION
In contrast to the human literature (2,4–6), but similar to the previous piglet study (1), we found no evidence of life-threatening chemoreflex-mediated responses after pharyngeal fluid stimulation during sleep in healthy term and preterm infants at term equivalent age.
Most surprising was the finding that pharyngeal fluid stimulation did not induce apnea. Our data indicate that the occurrence of apnea defined as ≥2 s (3) did not significantly change after pharyngeal infusion when compared with spontaneous (control) rates in both term and preterm infants. Several factors may account for these differences. The first, concerns preterm infants and may relate to the younger postmenstrual age of the infants in previous studies (3–6). In these studies the preterm infants were 27-36-wk postmenstrual age compared with term equivalent age in the present study. Another confounding variable may relate to the methodology. The reported "sham" or control stimuli were very short sleep epochs of 10 s undifferentiated as for sleep state (3,4,6) and thus do not adequately detect the high spontaneous occurrence of short sleep apnea and the apnea rate which is dependent on sleep state (Fig. 2, A and B).
Irrespective of such confounding variables, however, apneic episodes of ≥2 s could not be considered clinically significant. Data from the present study confirm that short apnea is a common and normal event in both term and preterm infants. Apneic events of longer duration (defined as ≥5 s, similar to our piglet study) (1) did not increase from baseline levels in response to pharyngeal fluid stimulation, and the duration of apneic events for both definitions did not change from that which occurs spontaneously.
In view of the absence of laryngeal-mediated responses such as apnea and bradycardia evoked in both human and animal studies (2,3–6,9,10,18,19), we propose that upper airway responses in term infants after pharyngeal fluid stimulation do not provide evidence of LCR stimulation but rather effective protection from such stimulation. Such protection, however, is dependent on intact swallowing. Similar reasoning suggests the same applies to our findings in preterm infants by term equivalent age. Such a conclusion, however, is not intended to negate the findings previously reported in preterm infants (4–6). On the contrary, comparison of responses evoked in preterm infants at term equivalent age in this study and preterm infants in the forementioned studies (4–6) may suggest a maturational improvement of airway defense in preterm infants with increasing postmenstrual age.
The most common response after pharyngeal infusion, in both AS and QS, was swallowing. Of particular interest, however, is the finding that spontaneous swallowing during sleep, in term and preterm infants, is dependent on sleep state and occurs more frequently in AS compared with QS (Table 1). The effect of sleep and sleep state on spontaneous swallowing has been reported in healthy adults (20–22) but not in healthy infants or children. The adult studies report the average spontaneous swallowing rates in sleep are 5.3 swallows/h (21) and 5.8 swallows/h (22). The rate of primary peristalsis (defined as immediately preceded by a pharyngeal swallow) for sleep state in adult subjects (mean ± SEM/h) is 2 ± 2 during rapid eye movement sleep and 1 ± 1 during stage III and IV sleep (20). Our data indicate that spontaneous swallowing rates/h during sleep in infants far exceeds that reported in the adult. Specifically, in AS, term and preterm infants swallow 78 and 96 times/h and in QS, 30 and 12 swallows/h, respectively. Spontaneous swallowing rates in infants during QS has also been reported in a group of infants under 1 y of age with symptoms suggestive of GER (23). Although the QS epochs were sleep-staged behaviorally and not electrophysiologically, spontaneous swallowing rates were reported as 0.45 swallow/min in patients with defined pathologic reflux and 0.46 swallow/min in "symptomatic controls."
Despite the effect of sleep state on spontaneous swallowing, the magnitude of the swallowing responses elicited after infusions into the pharynx were not significantly different between sleep states. Furthermore, the magnitude of the swallow response was similar between term and preterm infants. The only significant difference between the term and preterm infants was that preterm infants had a significantly greater swallow occurrence after infusion of medium bolus volumes (0.2 mL).
A volume-dependent response for swallowing rate has been reported in sleeping preterm infants after introduction of saline into the pharynx in sleep (3). The authors proposed that swallowing rate increases with larger volumes when the dependent piriform fossa is filled to capacity and begins to spill into the interarytenoid space where swallow receptors are located and triggered. In the present study, infusion of larger fluid boluses (0.35 and 0.2 mL) significantly increased swallow occurrence and frequency compared with the smaller volume (0.04 mL) in both term and preterm infants. These data therefore would also suggest such a volume-dependent swallow mechanism.
The failure to elicit any arousal or expiratory reflexes in QS is contrary to the results reported after direct laryngeal fluid stimulation (via a tracheostomy) in both sleeping adult dogs (10) and sleeping preterm and mature lambs (11,12). It is possible that the different arousal response in these animal studies may be attributed to the stimulus being evoked via different afferent inputs. In these animal studies, arousal from QS may have been evoked by direct stimulation of laryngeal receptors (10). In addition, arousal may result from hypoxemia secondary to LCR-induced apnea (10), as a strong correlation between arousal latency and apnea duration has been reported in QS (12). Furthermore, the authors of these studies suggest AS is the more vulnerable of the sleep states, because arousal responses were depressed in AS compared with QS (9,11). These studies suggest, therefore, that should the larynx be stimulated the response would be most exaggerated in AS. It may be therefore that arousal arising from pharyngeal fluid stimulation in AS in this study is indeed protective in that it precludes the laryngeal-induced apnea and bradycardia that may be evoked and would be prolonged in AS compared with QS (10,12,24). This is based on the failure to augment ventilation and the delayed arousal at low Sao2 during hypoxemic chemoreceptor stimulation in AS (24).
In conclusion, when supine airway defense during sleep in term infants and preterm infants who have reached term equivalent age is maintained after fluid stimulation of the pharynx. Similar to the piglet model, the primary mechanism of airway defense is swallowing.
Abbreviations
- LCR:
-
laryngeal chemoreflex
- SIDS:
-
sudden infant death syndrome
- AS:
-
active sleep
- QS:
-
quiet sleep
- Sao2:
-
oxygen saturation
- GER:
-
gastroesophageal reflux
References
Page M, Jeffery HE, Marks V, Post EJ, Wood AKW 1995 Mechanisms of airway protection after pharyngeal infusion in healthy, sleeping piglets. J Appl Physiol 78: 1942–1949
Lindgren C, Grogaard J 1996 Reflex apnoea response and inflammatory mediators in infants with respiratory tract infection. Acta Paediatr 85: 798–803.
Pickens DL, Schefft GL, Thach BT 1989 Pharyngeal fluid clearance and aspiration preventive mechanisms in sleeping infants. J Appl Physiol 66: 1164–1171
Davies AM, Koenig JS, Thach BT 1988 Upper airway chemoreflex responses to saline and water in preterm infants. J Appl Physiol 64: 1412–1420
Perkett EA, Vaughan RL 1982 Evidence for a laryngeal chemoreflex in some human preterm infants. Acta Paediatr Scand 71: 969–972
Pickens DL, Schefft G, Thach BT 1988 Prolonged apnea associated with upper airway protective reflexes in apnea of prematurity. Am Rev Respir Dis 137: 113–118
Lucier GE, Storey AT, Sessle BJ 1979 Effects of upper respiratory tract stimuli on neonatal respiration: reflex and single neuron analysis in the kitten. Biol Neonate 35: 82–89
Downing SE, Lee JC 1975 Laryngeal chemosensitivity: a possible mechanism for sudden infant death. Pediatrics 55: 640–649
Lee JC, Stoll BJ, Downing SE 1977 Properties of the laryngeal chemoreflex in neonatal piglets. Am J Physiol 2: R30–R36
Sullivan CE, Murphy E, Kozar LF, Phillipson EA 1978 Waking and ventilatory responses to laryngeal stimulation in sleeping dogs. J Appl Physiol Respir Environ Exercise Physiol 45: 681–689
Marchal F, Corke BC, Sundell H 1982 Reflex apnea from laryngeal chemostimulation in the sleeping premature lamb. Pediatr Res 16: 621–627
Marchal F, Crance JP, Arnould P 1986 Ventilatory and waking responses to laryngeal stimulation in sleeping mature lambs. Respir Physiol 63: 31–41
Kitchen WH 1983 Revised intrauterine growth curves for an Australian hospital population. Aust Paediatr J 19: 157–161
Ng P, Dear P 1990 The predictive value of a normal ultrasound scan in the preterm baby: A meta analysis. Acta Paediatr Scand 79: 286–291
Anders T, Emde R, Parmalee A 1971 A Manual of Standardized Terminology, Techniques and Criteria for Scoring of States of Sleep and Wakefulness in Newborn Infants. Brain Information Service, Los Angeles, pp 2–8.
Butcher-Puesch MC, Henderson-Smart DJ, Holley D, Lacey JL, Edwards DA 1985 Relation between apnoea duration and type and neurological status of preterm infants. Arch Dis Child 60: 953–958
Norusis MJ 1990 SPSS/PC+ 4.0. Chicago, pp B65–C36
Boggs DF, Bartlett DJ 1982 Chemical specificity of a laryngeal apneic reflex in puppies. J Appl Physiol 53: 455–462
Johnson P 1974 Laryngeal induced apnea. In: Robinson RR (eds) SIDS Proceedings of the Francis E Camps Symposium. Canada Foundation Study SIDS, Toronto, pp 231–242.
Dent J, Dodds WJ, Freidman RH, Sekiguchi T Hogan WJ, Arndorfer RC, Petrie, DJ 1980 Mechanism of gastroesophageal reflux in recumbent asymptomatic human subjects. J Clin Invest 65: 256–267
Lear CSC, Flanagan JB, Moorrees CFA 1965 The frequency of deglutition in man. Arch Oral Biol 10: 83–100
Lichter I, Muir RC 1975 The pattern of swallowing during sleep. Electroencephalogr Clin Neurophysiol 38: 427–432
Sondheimer JM 1989 Clearance of spontaneous gastro-oesophageal reflux in awake and sleeping infants. Gastroenterology 97: 821–826
Jeffery HE, Read DJC 1980 Ventilatory responses of newborn calves to progressive hypoxia in quiet and active sleep. J Appl Physiol 48: 892–895
Acknowledgements
The authors thank the mothers and infants for their participation in this study. We also thank the nursing staff of both the wards and neonatal intensive care unit of King George V Hospital, in particular, Angela Smith and the lactation consultants as well as Valerie Drayton and the Early Discharge Team. Thanks also to Dr. Crista Wocadlo for assistance with the statistical analysis, and to Danielle Ius and Justin Needs for their help with the infant studies.
Author information
Authors and Affiliations
Additional information
Supported by the National SIDS Council of Australia and The National Health and Medical Research Council.
Rights and permissions
About this article
Cite this article
Page, M., Jeffery, H. Airway Protection in Sleeping Infants in Response to Pharyngeal Fluid Stimulation in the Supine Position. Pediatr Res 44, 691–698 (1998). https://doi.org/10.1203/00006450-199811000-00011
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199811000-00011