Abstract
A sensitive nonisotopic immunoassay for the determination of 17-hydroxyprogesterone (17-OHP) levels in saliva was developed. The new time-resolved fluorometric immunoassay employs a specific polyclonal anti-17-OHP antiserum immobilized onto microtiter plates, a 17-OHP-biotin conjugate as a tracer, and streptavidin-europium a as secondary probe. The lower detection limit of the assay is 23.6 pmol/L (mean -3 s of a 22-fold zero determination) corresponding to 0.39 pg/well. The coefficients of intraassay variation are 8.8, 5.3, and 8.3% at the respective concentrations of 90.9, 454.5, and 1363.5 pmol/L. The coefficients of interassay variation are 8.8, 5.3, and 8.3% at the respective concentrations. Saliva was collected in commercially available devices. Reference ranges were established using 394 saliva samples from 132 healthy children, adolescents, and adults. Morning, midday, and evening levels of 17-OHP levels in saliva varied significantly in all age groups with morning levels being higher than midday and evening levels. Saliva samples (n = 57) were also obtained from 18 children with congenital adrenal hyperplasia (CAH). Salivary 17-OHP levels in the limited number of CAH patients studied ranged from 121 to 106 050 pmol/L. In conclusion 1) a new, sensitive nonisotopic immunoassay for measurement of 17-OHP in saliva has been developed; 2) reference ranges for healthy children, adolescents, and adults have been established; 3) there is a circadian pattern of 17-OHP levels in saliva at all ages; and 4) measurement of 17-OHP in saliva should be further evaluated over a longer period of time as a potentially reliable and powerful technique to monitor metabolic control in patients with CAH. As 17-OHP levels in saliva are stable for > 10 wk at 4°C, the technique is ideally suited for outpatient sampling.
Similar content being viewed by others
Main
Steroid 21-hydroxylase deficiency is the most frequent autosomal recessive genetic disorder in man (1,2). Untreated individuals who are homozygous for the disorder will experience virilization, precocious pseudopuberty, and in the salt-wasting CAH form, salt losing crisis, and dehydration (1). Patients who have bad metabolic control during infancy and adolescence will finally have reduced adult height. In addition, adult female subjects may have menstrual disturbances and infertility (3–5). 17-OHP, androstenedione, and 3α-androstandiolglucuronide serum levels, urinary steroids (4,6–8), and more recently salivary 17-OHP levels (9–25) are used to monitor metabolic control in children, adolescents, and adults with CAH.
It has been suggested that salivary 17-OHP levels actually correspond to free or the biologically active fraction of serum 17-OHP levels, because only low levels of sex hormone-binding globulin have been identified in human saliva (26–30). In addition, the ready availability of this fluid and the fact that the collection of saliva is a noninvasive and less stressful technique than blood sampling makes salivary steroid assays an attractive alternative to other, traditional methods of investigation requiring blood or urine collection (27,30,31). For example, the collection of saliva is more convenient and less cumbersome than the timed 12 or 24 h collection of urine. Most importantly in children, stress-related increases in 17-OHP are not encountered when saliva is assayed. As even repetitive salivary sampling has been shown to not induce increased release of cortisol, which is known to be the most prominent stress hormone (32), it is reasonable to assume that the approach of salivary sampling does not induce a release of 17-OHP either. Due to the rather low concentrations of 17-OHP in saliva, determination of the steroid has not always been easy and requires sensitive and highly specific assay techniques (9,10,12–14,32,33). Due to the variability in tracer preparation RIAs have the disadvantage of changing sensitivity and as a consequence a questionable reliability during day-to-day clinical application. We have therefore developed a new and sensitive nonisotopic immunoassay for the determination of 17-OHP levels in saliva. The new TR-FIA was validated, and normative data were generated in a group of healthy children, adolescents, and adults.
METHODS
Materials. 17-OHP-3CMO, dimethylformamide, dicyclohexylcarbodiimide, N-hydroxysuccinimide, 4-dimethylaminocinnamaldehyde spray reagent, bovine γ-globulin, BSA, and Tween 40 were purchased from Sigma Chemical Co., Deisenhofen, Germany. N-Biotinyl-1,8-diamino-3,6-dioxaoctan was from Boehringer Mannheim, Mannheim, Germany. 17-OHP was obtained from Fluka, Neu-Ulm, Germany. Potentially cross-reacting steroids were from Steraloids, Germany. All other reagents were of analytical grade and obtained from Merck, Darmstadt, Germany. Salivette® sampling devices were from Sarstedt, Numbrecht, Germany. Maxisorp® microtiter plates were purchased from Nunc, Roskilde, Denmark.
Saliva sampling. Saliva samples were collected before meals at 0800, 1200, and 1800 h. Saliva sampling devices consisting of a 40 × 9-mm polyester tampon with a polypropylene film, and an outer and inner tubing were used. Ten minutes after rinsing the mouth with water, the polyester tampon was chewed on for 5 min. The tampon was then transferred to the inner of the two polystyrene tubes. Saliva samples were stable at ambient temperature for at least 3 wk and at 4°C for more than 10 wk. The tubes were centrifuged for 10 min at 1800 × g to transfer the salivary liquid to the outer tube (34,35). Saliva samples which by visual inspection were contaminated by blood or debris were discarded and excluded from the analysis. Saliva samples were stored frozen at -20°C until analysis.
Synthesis of 17-OHP-biotin conjugate. An aliquot of 12.4 µmol of 17-OHP-3CMO was dissolved in 100 µL of dimethylformamide, and 112.4 µmol of both dicyclohexylcarbodiimide and N-hydroxysuccinimide, dissolved each in 50 µL of dimethylformamide, were added to form a 17-OHP-3CMO-N-hydroxysuccinimide ester derivative. The reaction mixture was stirred overnight at room temperature. Subsequently, 12.4 µmol of N-biotinyl-1,8-diamino-3,6-dioxaoctan were added in 100 µL of dimethylformamide and left for 3 h at room temperature.
Purification and characterization of 17-OHP-biotin conjugate. The tracer conjugate was isolated by preparative thin layer chromatography on Silica Gel 60 with methanol and chloroform (60/40, vol/vol) as the mobile phase. Retardation of the resolved spots was compared with that of 17-OHP-3CMO and of N-biotinyl-1,8-diamino-3,6-dioxaoctan using UV light at 254 nm and 4-dimethylamino-cinnamaldehyde as a specific dye for biotin moieties. The desired 17-OHP-biotin conjugate was scraped off the plate and extracted from the silica gel with 10 mL of ethanol. Rechromatography of the extract resulted in a single spot upon analysis by thin layer chromatography. Further analysis of the conjugate was performed by fast performance liquid chromatography (Pharmacia, PepRPC 10/20, 15 µm) (34).
17-OHP antiserum. The 17-OHP antiserum had been raised by repetitive immunization with 17-OHP-3CMO coupled to BSA as the immunogen. The specificity of the antiserum had been characterized before using a RIA technique (8). The specificity of the antiserum in the newly developed 17-OHP time-resolved fluorescence immunoassay was analyzed by determining the cross-reactivity of a large variety of related steroids.
Immunoassay design. A monoclonal mouse anti-rabbit IgG, 200 ng/well, generated in our laboratory, was immobilized as the capture antibody on microtiter plates in 200 µL of 50 mM sodium phosphate, pH 9.6. After a wash step, the plate was coated with the anti-17-OHP antiserum (200 µL; 1:50,000 dilution) in assay buffer, containing 0.5% BSA and 0.05% bovine γ-globulin, 50 mM Tris-HCl, 150 mM NaCl, 0.05% (wt/vol) NaN3, pH 7.7. Before the immunoassay procedure the plate was washed three times. Subsequently 50 µL of standard (diluted in artificial saliva consisting of 4.2 g/L NaHCO3, 0.5 g/L NaCl, and 0.2 g/L K2CO3, analogous to the German Industry Norm (DIN 53 160), or saliva, were pipetted together with 100 µL of 17-OHP-biotin (final dilution 1:5 000 000) into the microtiter wells. The plate was then incubated for 16-18 h at 4°C. The plate was washed three times, and 15 ng of streptavidin-europium were added in 200 µL of assay buffer and incubated for 30 min at ambient temperature on a horizontal shaker (31,34). After another 6-fold wash step, 300 µL of enhancement solution (Wallac Oy, Turku, Finland) were added to trans-chelate europium into a highly fluorescent complex. The signal was read in a Delfia® 1232 fluorometer (Wallac Oy, Turku, Finland).
Subjects and patients. Before the study written approval of the study protocol by the ethical committee of the University of Giessen, Medical Faculty, was obtained. A consent form and detailed explanations as to the nature of the study was handed out to each individual and/or their parents. One hundred thirty-two healthy children, adolescents, and adults (age 6 mo to 40 y, 68 male, 64 female) were recruited from among neighbors, friends, and families of the study coordinators. The rate of successful saliva sampling when carried out in a patient and supportive way even in children younger than 5 y of age was more than 90%. In fact, it is possible to even collect repetitive saliva samples from newborns and very young infants easily. Saliva samples were also obtained from 18 children with CAH who are being cared for by the Paediatric Endocrinology group at the Children's Hospital of the University of Giessen, Germany. Patients with CAH were between 2 and 18 y old (10 girls and 8 boys). Fourteen of the children had CAH of the simple virilizing form; four had CAH of the salt-loosing type. All children were given hydrocortisone and fludrocortisone replacement therapy but did not receive any other medication at the time of the study.
Urinary steroid measurements. Pregnanetriol and pregnanediol were measured in timed overnight urine collections from 14 children with CAH due to 21-hydroxylase deficiency (simple virilizing form) as described previously (6).
Statistical analysis. Data analysis was performed using the SPSS software program (SPSS Inc., Chicago, IL). t test and Spearman rank comparison were performed as was simple correlation analysis.
RESULTS
Isolation and characterization of the 17-OHP-biotin conjugate. As shown by thin layer chromatography (Fig. 1A) and 4-dimethylaminocinnamaldehyde staining (Fig. 1B), the conjugate separated well from unconjugated biotin and unconjugated 17-OHP. Analysis of the conjugate by fast performance liquid chromatography (Pharmacia, PepRPC 10/20, 15 µm) also showed complete separation of the conjugate from free biotin and 17-OHP (Fig. 2). When stored at -20°C at a dilution of 1:500 in ethanol, the conjugate has no been stable for several years (data not shown), enabling the use of the identical tracer preparation for many consecutive assay performances.
(A and B) Formation of the 17-OHP-3-CMO active ester and of the end product as monitored by thin layer chromatography. The solid phase was silica gel with an UV indicator (254 nm); the mobile phase consisted of a mixture of methanol:chloroform (60:40). Visualization of the compounds was carried out using a UV lamp. In B, 4-dimethylaminocinnamaldehyde was used as a specific dye for the ureido group of biotin (1, N-hydroxysuccinimide; 2, dicyclohexylcarbodiimide; 3, 17α-OH-progesterone-3-CMO; 4, 17α-OH-progesterone-3-CMO active ester; 5, 17α-OH-progesterone-3-CMO-biotin; 6, N-biotin-1,8-diaminodioxaoctan).
Analysis of 17-OHP-biotin-conjugate by fast performance liquid chromatography. A methanol gradient was established and a PepRPC 10/20, 15-µm column (Waters) was used. The elution fractions were traced at 254-nm UV light (upper panel) or analyzed by a commercially available RIA (Biermann, Germany) (lower panel).
Assay characteristics. In the time-resolved fluorometric immunoassay, the rabbit anti-17-OHP antiserum shows minor cross-reactivities of 4.3% with progesterone, 4.12% with 21-desoxycortisol, 1.15% with 11-desoxycortisol, and 0.35% with 17α-hydroxypregnenolone. All other steroids tested showed no cross-reactivity (displacement potency below 0.1%) (Table 1). The lower detection limit of the 17-OHP TR-FIA was determined by 22-fold measurement of the zero standard. The mean and the SD of the signals obtained were calculated, and the minimal detectable dose was defined as the concentration corresponding to the mean -3 SD signal (99% confidence interval). The newly developed 17-OHP TR-FLA showed a lower detection limit of 23.6 pM corresponding to 0.39 pg/tube. This low detection limit permits the use of as little as 50 µL of saliva per tube. Reproducibility of the measurements was tested by determination of their coefficient of variation for three saliva samples at concentrations of 90.9, 454.5, and 1363.5 pM. The intraassay coefficient of variation as determined by 13-fold analysis was 8.8, 5.3, and 8.3%, respectively. The interassay coefficient of variation as measured in nine independent assays was 11.7, 9.4, and 4.1% at the respective doses. Linearity of the measurements was demonstrated by serial dilutions (1:5 up to 1:160 in artificial saliva) of a sample from a patient with CAH and poor metabolic control displaying high salivary 17-OHP levels. The measured values were within a range of 92 to 112% of the expected value (mean 102%). Recovery was assessed by spiking saliva with 15 up to 758 pmol of 17-OHP standard. The recovery ranged from 100 to 128% of the expected values with a mean recovery of 112%.
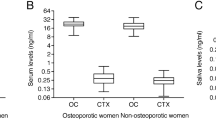
Normative data of 17-OHP levels in saliva. Reference ranges of 17-OHP levels in saliva were established using 394 saliva samples from 132 healthy children, adolescents, and adults. Morning, midday, and evening levels of 17-OHP levels in saliva varied significantly in all age groups with morning levels being higher than midday and evening levels. Figure 3 shows the 10th, 50th, and 90th centile for morning, midday (noon), and evening 17-OHP concentrations in saliva of the study population. Data were grouped according to age. There was no significant sex difference of 17-OHP levels in all age groups. However, significantly higher levels of 17-OHP were found in saliva samples from children under the age of 1 y than in saliva samples from subjects of any other age group (t test, p < 0.001). The lowest levels of 17-OHP were measured in saliva from prepubertal children in age groups 1-5, 6-10, and 11-15 y, whereas later in adolescence/puberty 17-OHP levels are again higher (Table 2). In all age groups there was a significant, circadian pattern of 17-OHP levels in saliva, with morning levels being highest and evening levels lowest. Absolute 17-OHP levels in adult women were somewhat lower than the values in male subjects; however, this difference was not significant (Fig. 3). In addition, the relatively large inter individual variations in the adult group could be explained by differences caused by variations throughout the cycle in the female group. Due to the limited number of subjects in this group this hypothesis could not be tested.
17-OHP levels in saliva from children with CHA. Saliva samples (n = 57) were also obtained from 18 children with CAH. Salivary 17-OHP levels in CAH patients ranged from 121 to 106 050 pmol/L and correlated with other biochemical (urinary pregnanetriol and pregnanediol, serum 17-OHP, and androstenedione) parameters of metabolic control in these children. As an example, Figure 4 shows the relationship between the 17-OHP concentration in saliva at noon (random sample) and the corresponding pregnanetriol levels in 12-h timed urine collection specimen from 14 children with CAH. There was a close relation between the urinary excretion of 17-OHP metabolite and the salivary 17-OHP levels (p < 0.0001, r = 0.94, y = 5.8 ± 0.6x - 275.1 ± 310.9).
DISCUSSION
The measurement of 17-OHP in saliva offers attractive advantages over alternative biochemical measurements during the care for patients with CAH (13–25). Most importantly, the collection of saliva is a noninvasive and less stressful technique than blood sampling (31,34). Therefore, stress-related increases in 17-OHP are unlikely to be encountered when saliva is assayed (32). Also, it can be envisioned that multiple determinations of 17-OHP in saliva during the day might enable the physician to actually tailor medication for an individual patient with CAH in respect to dosage and distribution of hydrocortisone toward a more physiologic way than what is accomplished when blood or urine chemistry is used to monitor metabolic control (18–20).
Despite all of these advantages and although the measurement of salivary 17-OHP had been pioneered by a number of groups (13–25), in many pediatric endocrinology clinics routine measurements of salivary 17-OHP levels are still not being performed. This is in one part due to the lack of sensitive and reliable immunoassays for the measurement of 17-OHP in saliva leading to relatively high sample volume requirements (or implying the need for an extraction step by organic solvent). Second and most importantly the clinical use was also compromised due to the scarcity of normative data on 17-OHP levels in healthy population, especially in the pediatric age group. In addition, many clinicians might wish to also measure plasma concentrations of renin and androstenedione in patients with CAH, particularly in salt-wasting syndrome. This might lead to a more frequent use of blood sampling and less frequent use of saliva sampling.
Due to the rather low concentrations of 17-OHP in saliva, determination of the steroid has not always been easy and requires sensitive and highly specific assay techniques (8,13,14,32). Due to variability in tracer preparation RIAs have the disadvantage of changing sensitivity and therefore questionable reliability during day-to-day clinical application. We have therefore developed a new and sensitive nonisotopic immunoassay for the determination of 17-OHP levels in saliva. The new TR-FIA was validated, and normative data were generated in a larger group of healthy children, adolescents, and adults. In general our data compare well with the 17-OHP levels that have been measured in saliva by other groups using conventional RIAs (13–25).
In conclusion 1) a new, sensitive nonisotopic immunoassay for measurement of 17-OHP in saliva has been developed; 2) reference ranges for healthy children, adolescents, and adults have been established; 3) there is a circadian pattern of 17-OHP levels in saliva at all ages; and 4) measurement of 17-OHP in saliva should be evaluated as a potentially reliable and powerful technique to monitor metabolic control in patients with CAH. As 17-OHP levels in saliva are stable for > 10 wk at 4°C, the technique is ideally suited for outpatient sampling. It is hoped that the routine application of this new assay will help to design treatment strategies to tailor hydrocortisone medication in individual CAH patients in a more physiologic way (36,37). This could be achieved during day-to-day routine life conditions in the home setting. In addition, as has been shown years ago, the determination of salivary 17-OHP levels can be used in screening programs to test for nonclassical 21-hydroxylase deficiency (10).
Abbreviations
- CAH:
-
congenital adrenal hyperplasia
- TR-FIA:
-
time-resolved fluorometric immunoassay
- 17-OHP:
-
17-hydroxyprogesterone
- 17-OHP-3CMO:
-
17-hydroxyprogesterone-3-O-carboxymethyloxime
References
New MI 1995 Steroid 21-hydroxylase deficiency (congenital adrenal hyperplasia). Am J Med 98: 2S–8S
White PC, New MI 1992 Genetic basis of endocrine disease: congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab 74: 6–11
Killeen AA, Hanson NQ, Eklund R, Vairl CJ, Eckfeldt JH 1992 Prevalence of nonclassical congenital adrenal hyperplasia among women self-referred for electrolytic treatment of hirsutism. Am J Med Genet 42: 197–200
James VH, Few JD 1985 Adrenocorticosteroids: chemistry, synthesis and disturbances in disease. Clin Endocrinol Metab 14: 867–892
Lim YJ, Batch JA, Warne GL 1995 Adrenal 21-hydroxylase deficiency in childhood: 25 years' experience. J Paediatr Child Health 31: 222–227
Rittner HL, Lee PDK, Blum WF, Doerr HG, Steiss J, Kreuder J, Rascher W, Kiess W 1997 Developmental patterns of serum 3α-androstandiol glucuronide. J Endocrinol Invest 20: 245–250
Otten BJ, Wellen JJ, Rijen JC, Stoelinga GB, Benraad TJ 1983 salivary and plasma androstenedione and 17-hydroxyprogesterone levels in congenital adrenal hyperplasia. J Clin Endocrinol Metab 57: 1150–1154
Von Schnakenburg K, Bidlingmaier F, Knorr D 1980 17-hydroxyprogesterone, androstenedione, and testosterone in normal children and in prepubertal patients with congenital adrenal hyperplasia. Eur J Pediatr 133: 259–267
Zerah M, Pang S, New MI 1987 Morning salivary 17-hydroxyprogesterone is a useful screening test for nonclassical 21-hydroxylase deficiency. J Clin Endocrinol Metab 65: 227–232
Zerah M, Ueshiba H, Wood E, Speiser PW, Crawford C, McDonald T, Pareira J, Gruen D, New MI 1990 Prevalence of nonclassical steroid 21-hydroxylase deficiency based on a morning salivary 17-OHP screening test: a small sample study. J Clin Endocrinol Metab 70: 1662–1667
Hampl R, Foretova L, Sulcova J, Starka L 1990 Daily profiles of salivary cortisol in hydrocortisone treated children with congenital adrenal hyperplasia. Eur J Pediatr 149: 232–234
Walker RF, Hughes IA, Riad-Fahmy D 1979 Salivary 17α-OHP in congenital adrenal hyperplasia. Clin Endocrinol 11: 631–637
Price DA, Astin MP, Chard CR, Addison GM 1979 Assay of hydroxyprogesterone in saliva. Lancet 2: 368
O'Rorke A, Kane MM, Gosling JP, Tallon DF, Fottrell PF 1994 Development and validation of a monoclonal antibody enzyme immunoassay for measuring progesterone in saliva. Clin Chem 40: 454–458
Shen SX, Young MC, Hinohosa-Sandoval M, Hughes IA 1989 17-OH-Progesterone response to acute dexamethasone administration in congenital adrenal hyperplasia. Horm Res 32: 136–141
Young MC, Robinson JA, Read GF, Riad-Fahmy D, Hughes IA 1988 17-OH-Progesterone rhythms in congenital adrenal hyperplasia. Arch Dis Child 63: 617–623
Nahoul K, Scholler R 1987 Comparison of saliva and plasma 17-hydroxyprogesterone time-course response to hCG administration in normal men. J Steroid Biochem 26: 251–257
Hoepffner W, Hubl W 1986 Studies on the diurnal variations of 17-hydroxyprogesterone in saliva by enzyme immunoassay in patients with congenital adrenal hyperplasia. Exp Clin Endocrinol 87: 189–194
Hughes IA, Days J, Robinson J, Walker RF, Riad-Fahmy D 1985 Monitoring treatment in congenital adrenal hyperplasia. Use of serial measurements of 17-OH-progesterone in plasma, capillary blood, and saliva. Ann NY Acad Sci 458: 193–202
Hughes IA, Read GF 1984 Control in congenital adrenal hyperplasia monitored by frequent saliva 17-OH-progesterone measurements. Horm Res 19: 77–85
Dyas J, Read GF, Guha-Maulik T, Hughes IA, Riad-Fahmy D 1984 A rapid assay for 17α-OH-progesterone in plasma, saliva and amniotic fluid using a magnetisable solid-phase antiserum. Ann Clin Biochem 21: 417–424
Price DA, Astin MP, Chard CR, Addison GM 1979 Assay of hydroxyprogesterone in saliva. Lancet 2: 368–369
Walker RF, Hughes IA, Riad-Fahmy D 1979 Salivary 17-α-OH-progesterone in congenital adrenal hyperplasia. Clin Endocrinol 11: 631–637
Walker RF, Read GF, Hughes IA, Riad-Fahmy D 1979 Salivary 17-α-OH-progesterone in congenital adrenal hyperplasia. Clin Chem 25: 542–545
Walker RF, Fahmy DR 1978 Radioimmunoassay of 17α-hydroxyprogesterone in whole saliva and parotid fluid of children with congenital hyperplasia. J Endocrinol 79: 64P–65P
Hammond GL, Langley MS 1986 Identification and measurement of SHBG and CBG in human saliva. Acta Endocrinol 112: 603–608
Price DA 1984 Salivary hormone levels in infants and children. Front Oral Physiol 5: 51–68
Vining RF, McGinley RA 1984 Flux of steroids between blood and saliva. Front Oral Physiol 5: 21–22
Luisi M, Franchi F 1984 Salivary steroid measurement. An alternative approach to plasma assays in assessing endocrine function. Front Oral Physiol 5: 124–154
Riad-Fahmy D, Read GF, Walker RF, Griffiths K 1982 Steroids in saliva for assessing endocrine function. Endocr Rev 3: 367–395
Kiess W, Meidert A, Dressendorfer RA, Schriever K, Kessler U, Konig A, Schwarz HP, Strasburger CJ 1995 Salivary cortisol levels throughout childhood and adolescence: relation with age, pubertal stage, and weight. Pediatr Res 37: 502–506
Kirschbaum C 1991 Cortisolmessung im Speichel-Eine Methode der Biologischen Psychologie. Verlag Hans Huber, Toronto, pp 32–70.
Ueshiba H, Zerah M, New MI 1994 Enzyme-linked immunosorbent assay (ELISA) method for screening of non-classical steroid 21-hydroxylase deficiency. Horm Metab Res 26: 43–45
Dressendorfer R, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ 1992 Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J Steroid Biochem Mol Biol 43: 683–692
Meulenberg EP, Hofman JA 1990 The effect of pretreatment of saliva on steroid hormone concentrations. J Clin Chem Clin Biochem 28: 923–928
Laue L, Merke DP, Jones JV, Barnes KM, Hill S, Cutler GB 1996 A preliminary study of flutamide, testolactone, and reduced hydrocortisone dose in the treatment of congenital adrenal hyperplasia. J Clin Endocrinol Metab 81: 3535–3539
Van Wyk JJ, Gunther DF, Ritzen EM, Wedell A, Cutler GB, Migeon CJ, New MI 1996 The use of adrenalectomy as a treatment for congenital adrenal hyperplasia. J Clin Endocrinol Metab 81: 3180–3190
Acknowledgements
The authors thank Christian Smit for help in collecting samples and Professor Dr. Wolfgang Rascher for his support. We express our sincere gratitude to the children and their families who participated in the study. We gratefully acknowledge the generous gift of saliva collecting devices (Salivette®) by Sarstedt, Numbrecht, Germany.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dressendorfer, R., Strasburger, C., Bidlingmaier, F. et al. Development of a Highly Sensitive Nonisotopic Immunoassay for the Determination of Salivary 17-Hydroxyprogesterone: Reference Ranges throughout Childhood and Adolescence. Pediatr Res 44, 650–655 (1998). https://doi.org/10.1203/00006450-199811000-00006
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199811000-00006
This article is cited by
-
17-Hydroxyprogesteron im Speichel
Monatsschrift Kinderheilkunde (2012)