Abstract
The purpose of this study was to begin to examine the influence of inhaled NO on O2 toxicity. The survival of Sprague-Dawley rats exposed to>95% O2, >95% O2 + 10 ppm NO, >95% O2 + 100 ppm NO, and >95% O2 + 3 ppm NO2 was determined. Survival at 120 h was 2/24 in >95% O2, 2/12 in >95% O2 + 10 ppm NO, and 1/12 in >95% O2 + 3 ppm NO2. Survival at 120 h was 21/30 in>95% O2 + 100 ppm NO (p < 0.01 compared with >95% O2). Three additional groups of rats were exposed for 60 h to: 21% O2, >95% O2, or >95% O2 + 100 ppm NO. The lungs were then assayed for total protein, reduced (GSH) and oxidized glutathione(GSSG), and 4-hydroxy-2(E)-nonenal. Both of the high O2 groups had significantly (p < 0.05) lower GSH/mg protein and GSH/GSSG ratios compared with the 21% O2 group. The >95% O2 group had a higher 4-hydroxy-2(E)-nonenal/mg of protein than either the 21% O2 group (p < 0.05), or the >95% O2 + 100 ppm NO group (p < 0.05 compared with >95% O2, not different from the 21% O2 group). Additional groups of rats were exposed to either 21% O2, >95% O2, or >95% O2 + 100 ppm NO for 0, 24, 48, and 60 h. The lungs were examined for neutrophil accumulation, which was increased at 60 h in the two groups exposed to >95% O2, but adding NO had no effect. Thus, the overall result was that 100 ppm inhaled NO improved the survival of rats in high O2.
Similar content being viewed by others
Main
Inhaled NO has been shown to be a selective and potent pulmonary vasodilator in both animal(1) and human(2) studies. It is currently being evaluated as a treatment for the pulmonary hypertension associated with persistent pulmonary hypertension of the newborn(3–5), acute respiratory distress syndrome(6), and after surgery for congenital heart disease(7). Patients with these conditions are often treated with elevated inspired O2, commonly approaching 100% O2, which can have deleterious side effects(8).
NO has been implicated as both a prooxidant and an antioxidant. One might anticipate, therefore, that the addition of NO in the presence of high inspired O2 might modify the overall response to the high O2 exposure. For example, high O2 increases superoxide production(9), and superoxide and NO react spontaneously to form peroxynitrite, which can be toxic(10, 11). Furthermore, oxygen and NO readily combine to form NO2, which can also be toxic(12–14). On the other hand, NO can react with lipid peroxyl radicals to prevent lipid peroxidation(10, 15), and this might help thwart the increase in lipid peroxidation associated with oxygen toxicity(8, 16). Furthermore, NO can inhibit neutrophil accumulation(17) and activation(18, 19). It has been shown that, when endogenous NO production was blocked in neonatal rats, which are relatively O2-tolerant with Nω-nitro-l-arginine methyl ester, significantly fewer survived exposure to >95% O2 compared with control rats(20), suggesting that endogenous NO has some protective effect.
Therefore, the present study was carried out to begin to examine the influence of adding NO to the inhaled gas with elevated Po2. As a first step, we determined the effect of NO on survival of rats in >95% O2. A second set of studies was carried out to determine the effect of NO on commonly measured biochemical and physiologic indices of hyperoxic lung injury. A third set of studies were carried out to determine the effect of NO on hyperoxia-induced lung neutrophil accumulation.
METHODS
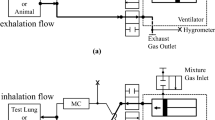
To expose rats to various gas mixtures, the rats (male Sprague-Dawley, 250-325 g body weight) were placed six at a time in a 44 × 22 × 20-cm Plexiglas exposure chamber. The gas within the chamber was continuously recirculated through a 1.5-L soda lime canister at 72 L/min (during the high O2 + NO2 exposure, the soda lime canister was empty). The>95% O2 atmosphere within the chamber was maintained by an O2 flow of approximately 10 L/min through the chamber. For the four exposure groups studied, the following gases were added to the O2 flow at the inlet to the chamber: 1) N2 at 100 mL/min designated“O2 alone” (n = 24, body weight = 312 ± 10 g); 2) 10 000 ppm NO in N2 at 10 mL/min designated“O2 + 10 ppm NO” (n = 12, body weight = 321± 8 g); 3) 10 000 ppm NO in N2 at 100 mL/min designated “O2 + 100 ppm NO” (n = 30, body weight= 285 ± 19 g); 4) 1000 ppm NO2 in N2 at 30 mL/min designated “O2 + 3 ppm NO2” (n = 12, body weight = 305 ± 10 g).
The last group was included because O2 and NO generate NO2, and thus, there was approximately 3 ppm NO2 in the exposure chamber of the high O2 plus 100 ppm NO group. The O2 (Beckman OM-14 oxygen analyzer), CO2 (Ciba-Corning blood gas analyzer model 278), and the NO and NO2 (electrochemical detector)(21) in the exposure chamber were measured at a minimum of every 6 h. The rats had access to food and water ad libitum. They were exposed to a 24-h cycle with 10-12 h of light, and the temperature in the exposure chamber ranged from 22.0 to 27.5 °C. To determine survival in each of the gas mixtures, the number surviving was tallied every 6 h for the 120 h of exposure.
Three additional groups of rats were exposed for 60 h to O2 alone(n = 10), to O2 plus 100 ppm NO (n = 11), or to room air (n = 10). After 60 h in the exposure chamber, the rats were anesthetized with sodium pentobarbital (50 mg/kg) i.p. A catheter was placed in a carotid artery and 1250 U/kg heparin was given. Blood was obtained for determination of hematocrit, and the rats were exsanguinated. The chests were opened, and the pleural fluid volume was aspirated and measured. A midline sternotomy was performed, and cannulas were placed in the pulmonary artery and the left atrium. The heart and lungs were removed en bloc and placed in a perfusion chamber. The lungs were perfused with 0.9% saline at 15 mL/min in a nonrecirculating manner until the venous effluent appeared to be clear of blood (3-5 min). During the perfusion, the lungs were ventilated with 15% O2, 5% CO2, and the balance N2 using a piston-type ventilator with a tidal volume (Vt) of 5 mL and an end-expiratory pressure of ≈0.1 kPa. The pulmonary arterial (Pa) and airway pressures (PA) were measured continuously during the perfusion. The dynamic compliance (Cdyn) was calculated as the Vt divided by the difference between the end-inspiratory and end-expiratory airway pressures. The lungs were then drained, weighed, frozen in liquid nitrogen, lyophilized, and stored at -70°C for later biochemical assay.
Three additional groups of rats were exposed to O2 alone, O2+ 100 ppm NO, or room air for 24, 48, or 60 h and in the case of O2 + 100 ppm NO for 120 h. After the exposures the rats were anesthetized with 50 mg/kg pentobarbital i.p., after which a catheter was placed in the trachea, and the lungs were infused with zinc formalin and held at 30 cm H2O for 16 h. After the inflation in formalin, the lungs were stored in 70% ethanol in preparation for paraffin embedding.
Glutathione assay. Ten milligrams of the finely ground lyophilized lung tissue were homogenized with 2 mL of 1 M HClO4 containing 2 mM EDTA and centrifuged at 5000 × g for 5 min at room temperature. The pH was brought to 6.6-6.8 with 2 M KOH containing 0.3 M 3-[N-morpholino]propanesulfinic acid and centrifuged at 1000 ×g for 5 min. The supernatant was then assayed for GSH and GSSG using the colorimetric enzymatic recycling assay as described by Tietze(22) and modified by Griffith(23). For the determination of GSH, the reagents 5,5′-dithiobis-(2-nitrobenzoic acid), NADPH, and glutathione reductase were obtained from Sigma Chemical Co. (St. Louis, MO) and dissolved in 0.1 M sodium phosphate buffer with 0.005 M EDTA at pH 7.5. The reagents were added to cuvettes in the following order: 5,5′-dithiobis-(2-nitrobenzoic acid)(0.58 μmol in 0.58 mL), 0.025 mL of sample, glutathione reductase (10 μg in 0.2 mL), and NADPH (0.2 μmol in 0.2 mL). The rate of 2-nitro-5-thiobenzoic acid formation in the sample was measured at 412 nm every 20 s for 5 min using a Gilson Response Spectrophotometer and compared with that obtained using known concentrations of glutathione. The GSSG in the sample was determined by derivitizing the sample GSH with 2-vinylpyridine before the recycling assay by adding 20 μL of 2-vinylpyridine to 1 mL of sample and incubating at room temperature for 1 h. Blanks included all reagents but no GSH standard or sample.
4-Hydroxynonenal assay. Ten milligrams of finely ground lyophilized lung tissue were homogenized on ice with 1 mL of ice-cold 20 mM Tris-HCl buffer at pH 7.4 and centrifuged at 3000 × g for 10 min at 4 °C. The supernatant was then assayed colorimetrically for 4-HNE using a commercially available kit (Lipid Peroxidation Assay Kit, catalog no. 437634, Calbiochem, La Jolla, CA). The assay is based on the formation of a stable chromophore from the reaction of N-methyl-2-phenylindole with 4 hydroxyalkenols at 45 °C. The N-methyl-2-phenylindole was diluted in acetonitrile to yield a 10 mM solution. Three volumes of the N- methyl-2-phenylindole were then diluted in 1 volume of 100% methanol. For the development of the chromophore, 200 μL of sample were added to 650 μL of the diluted N-methyl-2-phenylindole, tightly stoppered, mixed well, and incubated at 45 °C for 40 min. The tubes were then cooled, and the absorbance read at 586 nm using a Gilson Response Spectrophotometer. The concentration of 4-HNE was calculated by comparing absorbance values with those obtained using known concentrations of 4-HNE. Blanks consisted of buffer replacing the sample.
Total protein assay. Ten milligrams of finely ground lyophilized lung tissue were homogenized in 2 mL of 20 mM Tris-HCl buffer at pH 7.4 and centrifuged at 3000 × g for 10 min at room temperature. The supernatant was assayed colorimetrically for total protein using a commercially available assay (Bio-Rad). The assay is based on the Bradford(24) dye-binding procedure using Coomassie Brilliant Blue G-250. Ten microliters of sample were diluted in 790 μL of distilled water, and 200 μL of Bio-Rad reagent were added and mixed by inversion. Absorbance was read at 750 and 595 nm using a Gilson Response Spectrophotometer. The absorbance readings at 750 nm were subtracted from those at 595 nm and compared with values obtained from using known concentrations of BSA.
Neutrophil immunohistochemistry. Immunohistochemical analyses were performed on sections using a computer-driven CODE-ON system. Briefly, lung tissue sections were deparaffinated with a mixture of Hemo-De and xylene 3:1 (v/v) and rehydrated in PBS buffer (pH 7.3) containing 0.1% Tween 20. All reagents were prepared with a diluent that consisted of the PBS buffer/Tween supplemented with 0.5% BSA. To minimize background staining, sections were blocked with normal goat serum at room temperature for 20 min before application of the primary antibody. Blocking serum was removed, a rabbit anti-human MPO antibody was applied at a concentration of 1:3200, and sections were allowed to incubate in a moist chamber at room temperature overnight. Subsequently, sections were rinsed in the PBS/Tween buffer, then incubated at room temperature for 45 min with a biotinylated secondary antibody. Endogenous peroxidase activities were quenched with a combination of methanol and hydrogen peroxide. Sections were washed with the PBS/Tween buffer and incubated with avidin-biotin complex at room temperature for 45 min. The sections were incubated at room temperature for 7 min with diaminobenzidine, to which 1% nickel chloride was added, after which the sections were counterstained with eosin and mounted.
Neutrophil counting with computer assisted image analysis. Analyses were performed using Optimas imaging software on a Gateway 486/33 personal computer and a Summagraphics digitizing tablet. A color video camera was attached to an Olympus VANOX microscope that used a 20× objective lens with a 6.7× photo eyepiece. Live microscopic images were captured in a 24-bit true color frame grabber buffer, and the frozen images were displayed and analyzed on twin Sony monitors. Before image analysis, the system was calibrated with a stage micrometer to provide data in millimeters. The calibration was then saved to the system's configuration file.
Neutrophils were counted automatically by thresholding on the relatively low Gray scale value of immunohistochemically positive cells. As above, the macro provided the user with editing options to delete false positives and to add cells that were clearly immunoreactive. Neutrophil counts were expressed relative to alveolar area.
The number of neutrophils present in each of 10 random high power fields were counted in each section, and the alveolar surface area of the regions assessed were measured. The mean number of neutrophils per alveolar surface area was determined for each animal.
An interactive macro was written to automate data acquisition and export that data to a Microsoft EXCEL spreadsheet for analysis. The macro used the software's capability of thresholding on user-defined cellular or histologic structures, based on the structure's Gray scale value, which created screen objects that differentiated foreground from background. Alveolar area(mm2) and alveolar lengths (mm) were determined after setting threshold values to ensure the areas bounded by alveoli were foreground objects. For area determination, real number values were extracted from the area screen objects in calibrated units squared. The perimeter that bounds the area is a real number that is extracted from the area screen object, which gives the boundary length in calibrated units. The macro also tallied the number of screen objects created and the total was exported. Using the macro, the user was able to edit luminal structures such as vessels, or large airways, to ensure that only alveolar measurements were included for analysis.
Statistics. In the survival studies, data are presented as mean± SD. The percent animals surviving was compared among the three groups using the Fisher exact test. In the physiologic, biochemical, and lung neutrophil studies, data are presented as mean ± SE. In the physiologic and biochemical studies, the three groups were compared using ANOVA, followed by a Neuman-Keuls post hoc test. In the lung neutrophil accumulation studies, the groups were compared using a two-way ANOVA with time and treatment as factors. Subsequent analysis included a one-way ANOVA, followed by a Neuman-Keuls post hoc test. Differences were considered significant when p < 0.05.
RESULTS
The results of the survival studies are presented in Table 1 and Figure 1. The surviving fraction was significantly greater in the O2 + 100 ppm NO group (p < 0.005) than in any other group. The exposure time during which the mortality occurred (54-90 h) was similar for all four groups. The animals in all four groups appeared to be lethargic and dyspneic during this period. The survivors in all groups clearly became less lethargic and dyspneic after passing through this period. Although for practical reasons the exposure period was limited to 120 h, by 120 h all survivors appeared to have recovered, as indicated by what appeared to be normal activity and breathing patterns, although they had not returned to their initial body weight. The surviving rats had a significant weight loss from 291 ± 23 g to 251 ± 18 g (p < 0.001 by paired t test).
The survival curves of the four groups studied in the survival studies. The circles connected by the solid line represent the rats(n = 24) exposed to O2 alone, the triangles connected by the dashed-dotted line represent the rats (n = 12) exposed to O2+ 10 ppm NO, the triangles connected by the dashed line represent the rats(n = 30) exposed to O2 + 100 ppm NO, and the diamonds connected by the dotted line represent the rats (n = 12) exposed to O2 + 3 ppm NO2. The 120-h survival for the O2 + NO group was 70%, which was significantly (p < 0.005) higher than in the other three groups.
The physiologic variables measured at 60 h of exposure to either 21% O2, >95% O2, or >95% O2 + 100 ppm NO are summarized in Table 2. Both groups exposed to >95% O2 were significantly affected by the exposure, but there were no significant differences between the >95% O2 and >95% O2 + 100 ppm NO groups. In addition, the rats exposed to >95% O2 had a significant weight loss from 279 ± 4 g to 272 ± 4 g (p < 0.005 by paired t test); similarly the rats exposed to >95% O2 + 100 ppm NO had a significant weight loss from 303 ± 6 g to 286 ± 5 g (p < 0.005).
Both total glutathione/mg of protein and GSH/mg of protein were significantly less in the rats exposed to high O2 compared with room air rats. The rats exposed to >95% O2 + NO had lower total glutathione/mg of protein compared with rats exposed to >95% O2 alone (Table 3); however, there was no difference in the total glutathione/lung (Table 3). The normalization independent ratio of GSH/GSSG was significantly lower in the two groups of rats exposed to high O2 compared with room air rats. However, there was no difference in the GSH/GSSG ratio between the two groups exposed to high O2 (Table 3).
The 4-HNE/mg of protein and the 4-HNE/lung were significantly higher in the rats exposed to >95% O2 alone compared with controls(Table 3). In the rats exposed to >95% O2 + NO, the 4-HNE/mg of protein was not significantly different from nonexposed rats(Table 3), and the 4-HNE/lung was not significantly different from rats exposed to >95% O2 alone.
Lung neutrophil accumulation as a function of duration of exposure to>95% O2 with or without concomitant exposure to 100 ppm of NO is illustrated in Figure 2. There was an increase in lung neutrophils in the alveolar region as a function of exposure time, with no additional effect of O2 + 100 ppm NO over O2 alone. After 60 h of exposure, lung neutrophil accumulation was greater in the groups exposed to O2 or O2 + 100 ppm of NO, whereas at the 120-h time point the lung neutrophil counts were still significantly greater than at 0, 24, or 48 h of exposure. In the >95% O2 + 100 ppm NO group at 120 h of exposure there was no pleural effusion, whereas at 48 and 60 h of exposure there was 4.5 ± 1.5 mL and 6.9 ± 1.2 mL, respectively. This did not differ from the >95% O2 group which had 5.3 ± 1.4 mL at 60 h of exposure.
Lung neutrophil counts in alveolar regions were determined as a function of time exposed to O2 alone or O2 + 100 ppm NO. Alveolar neutrophils were higher at 60 and 120 h of exposure to O2 than at all earlier time points. There was no effect of NO at any duration of exposure to O2. Data are expressed as means ± SE,n = 4-6 in each group. *p < 0.05 compared with 0, 24, and 48 h of exposure.
DISCUSSION
That rats succumb to exposure to >95% O2 has been demonstrated repeatedly in the past(9, 12, 20, 25–33). The survival curve for the rats in the present study appears to be typical of rats in several previous studies(12, 16, 29). A variety of treatments have been found to have a protective effect. These include preexposure to 85% O2 for 3-7 d(12, 16, 26, 29), the i.v. injection of catalase and superoxide dismutase(31, 33), and the intratracheal administration of erythrocytes(32), superoxide dismutase(27), exogenous surfactant(34), endotoxin(28), or tumor necrosis factor(30). From the present study 100 ppm NO can apparently be added to this list.
Although it is not entirely clear how the various effects of high O2(e.g. gas exchange deficits due to edema, atelectasis, and/or restrictive impairment due to pleural effusion) combine to actually cause the death of the animals, the oxidative injury to the lung, particularly the pulmonary endothelium, is considered to limit survival. In the present study, the manifestations of lung injury such as pleural effusion, increased lung weight, decreased compliance, and so forth, measured at 60 h of exposure were consistent with severe lung injury. However, they did not distinguish between the groups with and without 100 ppm NO, and all the animals were clearly having respiratory distress in the 50-100-h period whether they went on to survive or not. Sixty hours was chosen for the physiologic and biochemical measurements because the animals exhibit the manifestations of lung injury, but it is before the precipitous fall in survival occurs.
Although the rats exposed to high O2 + 100 ppm NO had greater survival than rats exposed to high O2 alone, within the set of measurements made, only the 4-HNE/mg of protein appears to distinguish the inclusion of NO in the high O2 environment. It should be kept in mind that the lungs exposed to high O2 have a different cellular composition than do lungs exposed to room air(8, 16, 26). Thus, it is difficult to determine the appropriate normalization for the biochemical markers in these whole lung experiments. Previous studies have used both the amount of protein in the lung(25) and that normalized to the whole lung(33). With the caveat of normalization in mind, the 4-HNE/mg of protein results are consistent with the hypothesis that NO had an anti-lipid peroxidation effect in the lungs. It has been found that the oxidation of LDL can be inhibited by NO via a chain-breaking antioxidant mechanism(10, 15).
NO can also have antiinflammatory effects through inhibition of neutrophil adhesion(17), accumulation(19), as well as activation responses(17). However, the fact that inhaled NO did not lead to a detectable difference in lung neutrophil accumulation in the alveolar region does not provide any reason to believe that an effect on neutrophil adhesion was a contributing factor. Because activated neutrophils generate superoxide, the role of exogenous NO in inhibiting neutrophil activation and/or neutrophil-mediated lipid peroxidation remains to be elucidated and it may be found to be a contributing factor to the decreased 4-HNE found in the lungs from rats exposed to hyperoxia and inhaled NO.
There is previously reported evidence that inhaled NO is protective in oxidative lung injury. In the isolated perfused rabbit lungs, inhaled NO prevented the increase in permeability caused by oxidant injury induced by either H2O2(35) or by O2-(36). In isolated perfused rat lungs(37), it has been found that inhaled NO significantly reduced capillary leak induced by perfusion with N- formyl-methionyl-leucyl-phenylalanine and neutrophils. Thus, these studies might suggest that the protective effect of inhaled NO in hyperoxic exposure involved attenuation of the hyperoxia-induced increased permeability. However, the data in Table 2 do nothing to support such a conclusion, because the lung wet and dry weights were not different between the O2-exposed group and the O2 + NO group at 60 h of exposure. Other possibilities exist, for example, atelectasis with resultant mismatching of ventilation and perfusion may contribute to the hypoxemia of O2 toxicity(8), and inhaled NO has been found to improve ventilation-perfusion matching(38).
The effect of inhaled NO on survival of rats in hyperoxia has been reported in two previous studies. Gutierrez et al.(39) found that the addition of 7.8 ± 0.2 ppm NO to >95% O2 increased survival at 144 h. Garat et al.(40) could find no effect of either 10 ppm NO or 100 ppm NO on survival in >95% O2. Garat et al.(40) also reported that, in rats exposed to >95% O2 for 40 h, adding 100 ppm NO made no difference in the lung wet to dry weight ratios, or the appearance of i.v. administered 131I-labeled albumin in the alveoli. However, adding only 10 ppm NO decreased both lung wet to dry weight ratios and the appearance of i.v. administered 131I in the alveoli. The reason why the survival results differ in these studies and from those presented here is not clear. Previously reported strain-dependent differences in rat susceptibility to hyperoxia(41) is one possibility, Garat et al.(40) used Wistar rats, and Sprague-Dawley rats were used by both Gutierrez et al.(39) and in the current study.
In the presence of oxygen, NO is spontaneously oxidized to form NO2(14, 42). Therefore, it is difficult to maintain NO in a high O2 environment without the presence of some level of NO2. In this study we used a fairly high rate of turnover(approximately two-chamber volumes/min) and continuously recirculated the gas through soda lime to scrub NO2. We still had an NO2 level of about 3 ppm in the O2 + 100 ppm group. Thus, we felt it necessary to carry out the NO2 control. NO2 would not be expected to have a protective effect, because it is itself a toxic gas. For example, it has been previously found that rats exposed to 75 ppm NO2 in 21% O2 for 6 h had 100% mortality(12), and biochemical and morphologic evidence of lung injury has been detected in rats exposed to 25 ppm NO2 in 21% O2 for 30 min(14), and to 10 ppm NO2 in 21% O2 for 72 h(13). Thus, considering the toxic effects of NO2 on lungs in 21% O2 it was conceivable that even small amounts of NO2 might worsen survival of rats in high O2 or high O2 plus NO exposure. However, we found no obvious deleterious effects of 3 ppm NO2 on survival of rats with high O2 exposure.
Although it will take a much more extensive effort to define the mechanisms involved, the improved survival in the high O2 environment to which NO was added is a striking observation that appears to warrant further investigation. Including 100 ppm NO in >95% O2 resulted in a smaller increase in the lipid peroxidation product than that in high O2 alone. Therefore, it may be that the mechanism of improved survival of rats in high O2 with 100 ppm NO includes lower lipid peroxidation. On the other hand, the lower total glutathione levels in the O2 + NO group suggests that the overall impact of inhaled NO on oxygen toxicity is more complicated or specific than an across the board antioxidant effect. The main conclusions at this point are that inhaled NO increased survival in high O2 exposure and this type of exposure in the rat may provide a useful tool for examining certain aspects of the impact of NO on oxygen toxicity in future studies. The impact of adding NO to high inspired O2 is clinically relevant because many patients with various forms of acute lung injury, such as adult respiratory distress syndrome, persistent pulmonary hypertension of the newborn caused by meconium aspiration, and so forth, are being treated with inhaled NO while receiving very high fractions of inspired O2.
Abbreviations
- NO:
-
nitric oxide
- Pa:
-
pulmonary artery pressure
- Pv:
-
left atrial pressure
- Vt:
-
tidal volume
- Cdyn:
-
dynamic compliance
- GSH:
-
reduced glutathione
- GSSG:
-
oxidized glutathione
- 4-HNE:
-
4-hydroxy-2(E)-nonenal
References
Nelin LD, Moshin J, Thomas CJ, Sasidharan P, Dawson CA 1994 The effect of inhaled nitric oxide on the pulmonary circulation of the neonatal pig. Pediatr Res 35: 20–24.
Pepke-Zaba J, Higenbottam TW, Dinh-Xuan AT, Wallwork J 1991 Inhaled nitric oxide as a cause of selective pulmonary vasodilation in pulmonary hypertension. Lancet 338: 1173–1174.
Hoffman GM, Ross RA, Day SE, Rice TB, Nelin LD 1997 Inhaled nitric oxide reduces the utilization of extracorporeal membrane oxygenation in persistent pulmonary hypertension of the newborn. Crit Care Med 25: 352–359.
The Inhaled Nitric Oxide Study Group 1997 Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. N Engl J Med 336: 605–610.
The Neonatal Inhaled Nitric Oxide Study Group 1997 Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. N Engl J Med 336: 597–604.
Bigatello LM, Hurford WE, Kacmared RM, Roberts JD, Zapol WM 1994 Prolonged inhalation of low concentrations of nitric oxide in patients with severe adult respiratory distress syndrome. Anesthesiology 80: 761–770.
Roberts JD, Lang P, Bigatello LM, Vlahakes GJ, Zapol WM 1993 Inhaled nitric oxide in congenital heart disease. Circulation 87: 447–453.
Jackson RM 1990 Molecular, pharmacologic and clinical aspects of oxygen-induced lung injury. Clin Chest Med 11: 73–86.
Freeman BA, Crapo JD 1981 Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem 256: 10986–10992.
Hogg N, Kalyanaraman B, Joseph J, Struck A, Parthasarathy S 1993 Inhibition of low-density lipoprotein oxidation by nitric oxide: potential role in atherogenesis. FEBS Lett 334: 170–174.
Radi R, Beckman JS, Bush KM, Freeman BA 1991 Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys 288: 481–487.
Crapo JD, Sjostrom K, Drew RT 1978 Tolerance and cross-tolerance using NO2 and O2. I. toxicology and biochemistry. J Appl Physiol 44: 364–369.
Müller B, Barth P, Von Wichert P 1992 Structural and functional impairment of surfactant protein A after exposure to nitrogen dioxide in rats. Am J Physiol 263:L177–L184.
Stavert DM, Lehnert BE 1990 Nitric oxide and nitrogen dioxide as inducers of acute pulmonary injury when inhaled at relatively high concentrations for brief periods. Inhal Toxicol 2: 53–67.
Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, Kirk M, Freeman BA 1994 Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. J Biol Chem 269: 26066–26075.
Rosenbaum RM, Wittner M, Lenger M 1969 Mitochondrial and other ultrastructural changes in great alveolar cells of oxygen-adapted and poisoned rats. Lab Invest 20: 516–528.
Kubes P, Suzuki M, Granger DN 1991 Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA 88: 4651–4655.
Clancy RR, Leszczynska-Pisiak J, Abranson SV 1992 Nitric Oxide, an endothelial cell relaxation factor, inhibits neutrophil superoxide anion production via a direct action on the NADPH oxidase. J Clin Invest 90: 1116–1121.
Kubes P, Kanwar S, Niu XF, Gaboury JP 1993 Nitric oxide synthesis inhibition induces leukocyte adhesion via superoxide and mast cells. FASEB J 7: 1293–1299.
Pierce MR, Voelker CA, Sosenko IRS, Bustamante S, Olister SM, Zhang XJ, Clark DA, Miller MJS 1995 Nitric oxide synthase inhibition decreases tolerance to hyperoxia in newborn rats. Mediat Inflam 4: 431–436.
Nelin LD, Christman NT, Morrisey JF, Dawson CA 1996 Electrochemical nitric oxide and nitrogen dioxide analyzer for use with inhaled nitric oxide. J Appl Physiol 81: 1423–1429.
Tietze F 1969 Enzymic method for quantitiative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 27: 502–522.
Griffith OW 1980 Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106: 207–212.
Bradford MM 1976 A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254.
Canada AT, Herman LA, Young SL 1995 An age-related difference in hyperoxia lethality: role of lung antioxidant defense mechanisms. Am J Physiol 268:L539–L545.
Crapo JD, Barry BE, Foscue HA, Shelburne J 1980 Structural and biochemical changes in rat lungs occurring during exposure to lethal and adaptive doses of oxygen. Am Rev Respir Dis 122: 123–143.
Crapo JD, Tierney DF 1974 Superoxide dismutase and pulmonary oxygen toxicity. Am J Physiol 226: 1401–1407.
Tang G, Berg JT, White JE, Lumb PD, Lee CY, Tsan MF 1994 Protection against oxygen toxicity by tracheal insufflation of endotoxin: role of MnSOD and alveolar macrophages. Am J Physiol 266:L38–L45.
Tierney DF, Ayers L, Kasuyama RS 1977 Altered sensitivity to oxygen toxicity. Am Rev Respir Dis 117:S59–S65.
Tsan MF, White JE, Santana TA, Lee CY 1990 Tracheal instillation of tumor necrosis factor protects rats against oxygen toxicity. J Appl Physiol 68: 1211–1219.
Turrens JF, Crapo JD, Freeman BA 1984 Protection against oxygen toxicity by intravenous injection of liposome-entrapped catalase and superoxide dismutase. J Clin Invest 73: 87–95.
Van Asbeck BS, Hoidal J, Vercellotti GM, Schwartz BA, Moldow CF, Jacob HS 1985 Protection against lethal hyperoxia by tracheal insufflation of erythrocytes: role of red cell glutathione. Science 227: 756–759.
White CW, Jackson JW, Abuchowski A, Kazo GM, Mimmack RF, Berger EM, Freeman BA, McCord JM, Repine JE 1989 Polyethylene glycol-attached antioxidant enzymes decrease pulmonary oxygen toxicity in rats. J Appl Physiol 66: 584–590.
Ghio AJ, Facica PJ, Young SL, Piantadosi CA 1994 Synthetic surfactant scavenges oxidants and protects against hyperoxic lung injury. J Appl Physiol 77: 1217–1223.
Poss WB, Timmons OD, Farrukh IS, Hoidal JR, Michael JR 1995 Inhaled nitric oxide prevents the increase in pulmonary vascular permeability caused by hydrogen peroxide. J Appl Physiol 79: 886–891.
Kavanagh BP, Mouchawar A, Goldsmith J, Pearl RG 1994 Effects of inhaled NO and inhibition of endogenous NO synthesis in oxidant-induced acute lung injury. J Appl Physiol 76: 1324–1329.
Guidot DM, Repine MJ, Hybertson BM, Repine JE 1995 Inhaled nitric oxide prevents neutrophil-mediated, oxygen radical-dependent leak in isolated rat lungs. Am J Physiol 269:L2–L5.
Pison U, Lopez FA, Heidelmeyer CF, Rossaint R, Falke KJ 1993 Inhaled nitric oxide reverses hypoxic pulmonary vasoconstriction without impairing gas exchange. J Appl Physiol 74: 1287–1292.
Gutierrez HH, Nieves B, Chumley P, Rivera A, Freeman BA 1996 Nitric oxide regulation of superoxide-dependent lung injury: oxidant-protective actions of endogenously produced and exogenously administered nitric oxide. Free Radic Biol Med 21: 43–52.
Garat C, Jayr C, Eddahibi S, Laffon M, Meignan M, Adnot S 1997 Effects of inhaled nitric oxide or inhibition of endogenous nitric oxide formation on hyperoxic lung injury. Am J Respir Crit Care Med 155: 1957–1964.
Stenzel JD, Welty SE, Benzick AE, Smith EO, Smith CV, Hansen TN 1993 Hyperoxic lung injury in Fischer-344 and Sprague-Dawley rats in vivo. Free Radic Biol Med 14: 531–539.
Zapol WM, Rimar S, Gills N, Marletta M, Bosken CH 1994 Nitric oxide and the lung. Am J Respir Crit Care Med 149: 1375–1380.
Author information
Authors and Affiliations
Additional information
Supported by a grant from the American Heart Association of Wisconsin and the Department of Veterans Affairs. This work was done during the tenure of a Clinician-Scientist Award from the American Heart Association to L.D.N.
Rights and permissions
About this article
Cite this article
Nelin, L., Welty, S., Morrisey, J. et al. Nitric Oxide Increases the Survival of Rats with a High Oxygen Exposure. Pediatr Res 43, 727–732 (1998). https://doi.org/10.1203/00006450-199806000-00003
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199806000-00003