Abstract
In the doubly labeled water (DLW) method, for measurement of energy expenditure in humans, calculation of carbon dioxide production rate is influenced by the ratio (R) of the dilution spaces of the tracer isotopes 2H and 18O. Recent publications have recommended use of a fixed value for R, using a mean population value([horizontal bar over]R) rather than individually derived experimental values. However, most of the data considered previously were derived from adults. Infants are characterized by rapid rates of energy deposition and high body water content, either of which could influence isotope kinetics. We have considered whether use of a fixed R value is suitable for DLW studies of infants, and whether the adult [horizontal bar over]R value is appropriate. Data from 281 DLW measurements in healthy infants aged 6 wk to 12 mo were analyzed. Mean R of all measurements was 1.036 (SD 0.019), very similar to [horizontal bar over]R values recommended for adults. We found analytical precision could account for almost all between-subject variation at a single time point. However, we also found relationships between R and age and weight gain, indicating that physiology does influence the space ratio. The effect of weight gain on R was negligible, but changes of [horizontal bar over]R with age, reflecting changes in percentage body water during infancy, were of sufficient magnitude to affect calculation of energy expenditure significantly. Our analysis suggests that use of a fixed ratio at a single time point in infancy is acceptable, but that the appropriate value of -R changes over the first year of life.
Similar content being viewed by others
Main
TBW has been measured by an isotopic dilution techniques for over half a century(1), based on the principle that both the hydrogen atoms and the oxygen atom of the body water pool are fully exchangeable with water containing 2H or 18O atoms(2). The model assumes that the dilution spaces of these tracers (Nd or No are equal to TBW, but in practice both 2H and18 O also exchange with hydrogen and oxygen in biologic material. The extent of this process, sometimes termed nonaqueous exchange(2), varies between species(3), but is thought in humans to be approximately 4% for 2H and approximately 1% for 18O(4). Consequently, Nd and No overestimate TBW, and they do so by different amounts, the difference between them being described by the dilution space ratio,R.
Interindividual variability in R may in turn influence the accuracy of the doubly labeled water method, as has been considered previously(4–6). This method estimates carbon dioxide production, and hence total energy expenditure, from the turnover of 2H and 18O over several days. Isotope dilution spaces are used in the calculation of flow rates, and variation in R therefore affects the calculation of carbon dioxide production.
The issue is of particular importance for studies of younger subjects. First, noninvasive isotopic techniques are the only viable tool for assessing total energy expenditure in free-living infants and young children. Second, there is some evidence that R is lower than the adult value in very young infants(7–9). Third, infants are characterized by high rates of energy deposition and high body water content in comparison with adults, and these factors may influence R(8). It has been argued with respect to adults that because most variation in R arises from analytical error rather than physiology, a mean population value ([horizontal bar over]R) is more appropriate than individual experimental values(4, 5), provided that the appropriate value of [horizontal bar over]R is known(6). However, whether this is true for infants, and whether the appropriate [horizontal bar over]R value is the same for infants as for adults has not previously been considered in a large sample.
We have therefore evaluated the contribution of analytical variation and physiology to interindividual variation in R, using a data set comprising 281 DLW measurements in infants aged 6 wk to 12 mo. These data include 135 measurements summarized previously(10), together with an additional 146 measurements completed more recently.
METHODS
A total of 187 infants were recruited into four separate studies, one longitudinal and three cross-sectional, from the Rosie Maternity Hospital, Cambridge, UK. All infants were healthy, born at term, and had no known condition that might unduly influence health or development. Ethical permission for the studies was granted by the Cambridge Health Authority and the Medical Research Council's Dunn Nutrition Unit.
The infants were studied at 6 and 12 wk, and 6, 9, and 12 mo of age. The groups comprised 50 infants at 6 wk, 149 at 12 wk, 37 at 6 mo, 41 at 9 mo, and 19 at 12 mo. At each study period, a number of measurements were made over an 8-d period. Nude weight was measured using Seca 724 electronic scales (CMS Weighing Ltd, London, UK), accurate to 20 g. Skinfold thicknesses were measured at the triceps and subscapular sites using Holtain calipers, and supine length using a Harpenden Infantometer (Holtain, Dyfed, UK). Weight was measured again on the last day of the study period, allowing weight gain over 7 d to be calculated.
Energy expenditure and body composition were estimated through the DLW method, the methodologic principles of which are described in detail elsewhere(11). In this study, the isotopes were administered orally either as water or added to approximately 10 mL of made up formula milk. The dose was calculated according to the body weight of the infant, and was intended to give 0.28 g/kg of body weight H218O (10 atom% excess, Europa Scientific, Crewe, UK) and 0.10 g/kg of body weight2 H2O (99.9 atom% excess, Sigma Chemical Co. Chemicals, Poole, UK). A 1.5-mL sample of each dosing mixture was retained for mass spectrometer analysis. The dosing apparatus was weighed accurate to 2 decimal places of a gram before and after dosing, allowing the quantity of dose given to be determined. Urine samples were obtained from the infants before dosing and daily for the following 7 d using the method of leaving cotton wool balls inside the diaper of the infant and, after urination, obtaining the urine sample by inserting the cotton wool in a syringe(12). The parents were asked to check the diaper frequently for urination (at least once per hour), and the time of voiding was taken as the midpoint between the last two times of checking. The maximum error for the timing of the urine sample was therefore 30 min.
Urine samples and dosing mixtures were analyzed using an Aqua-SIRA dual mass spectrometer system (VG Isogas, Cheshire, UK) for simultaneous analysis of 18O/16O and 2H/1H isotope ratios in water(13). Each sample was analyzed nine times using consecutive 5-μL injections to overcome memory effects in the mass spectrometer. Samples were also analyzed in duplicate.
Isotopic enrichments are expressed as delta per million (δ) relative to the International Standard Vienna Standard Mean Ocean Water (SMOW), calculated as: Equation where Rs and RSMOW are the heavy:light isotope ratios in a sample and SMOW, respectively.
Tracer dilution spaces were calculated using the back extrapolation method(10), which has been shown to give significantly lower values for TBW than the plateau method in infants(14). The dilution space of each isotope at the beginning of the study period was calculated as Equation (1) where N is the dilution space, A is the dose given to the infant, T is the amount of tap water in which the dose sample was diluted, and a is the portion of the dose diluted for mass spectrometric analysis. N, T, a, and A are in grams. Ea is the isotopic enrichment of the dosing portion, Et that of tap water, and E s - Ep is the time 0 intercept of the log plot of body water enrichments (Es) net of predose(Ep) with time.


Average TBW was calculated as the mean of No/1.01 and Nd/1.04(4). Percentage body water was then calculated by dividing by weight on d 1. Both No and Nd, expressed as a percentage of body weight, were also used as variables in subsequent analysis. Fat-free mass was not calculated, to avoid assumptions concerning the hydration of lean tissue in early life.
Statistical analysis. The contributions of analytical precision and physiology were first assessed in the 141 infants measured successfully at 12 wk of age. Analytical precision was assessed using the values given in Table 1, which were determined from duplicate analyses of urine and dose samples. These values were derived from a subsample of 42 infants and have been assumed to be representative of all 141 subjects at 12 wk. Error propagation of analytical precision was undertaken as follows.Equation 1 was partially differentiated with respect to each of the terms Ea, Et,Es, and Ep, and then the expectations squared were derived according to a procedure described previously(15). The same methodology was applied to the space ratio. The relationship between R and physiologic variables was assessed through correlation analyses.
The effect of age was considered through two approaches. First, R was compared in 35 infants measured both at 6 wk and 6 mo of age, using a paired t test. Second, correlations between R and physiologic variables were investigated within the entire age range. Significantly correlating variables were then considered together using multiple regression analysis. All analyses were conducted using Minitab 1985 software.
RESULTS
Successful dosings were achieved in 49 infants at 6 wk of age, 141 at 12 wk, 37 at 6 mo, 36 at 9 mo, and 18 at 12 mo, giving a total of 281 data points(120 male, 161 female) for analysis. All tables refer to these successfully dosed infants. Unsuccessful dosings occurred when the infant vomited or regurgitated an unknown quantity of dose within 4 h of being dosed, or very rarely due to poor parental compliance in the collection of urine samples.
Age, anthropometry, and space ratio of the entire sample are given by age group in Table 2. Mean (±SD) space ratio of the entire sample was 1.036 ± 0.019. There was no significant difference in space ratio between the sexes (male, 1.036 ± 0.020; female, 1.036± 0.018).
Propagation of error analysis gave a SD for R of 0.018. This value was not significantly different from that of 0.019 derived by measurement from the sample of 141 infants at 12 wk. Correlation analyses showed that, at this time point, weight gain was significantly and positively related to R (p < 0.05), whereas skinfold thicknesses, percentage of body water, and weight were not (Table 3). There was no significant difference between the sexes at 12 wk (male,n = 64, 1.036 ± 0.19; female, n = 77, 1.041± 0.18; t = 1.53, p = 0.13).
Within the entire sample, infant age, weight, and weight gain were significantly related to R, as is shown in Table 4, whereas percentage body water and s.c. fatness were not related to R. When the three variables related to R were entered into a multiple regression equation, weight was no longer significant and was withdrawn from the model. Age remained negatively correlated with R, whereas weight gain was positively correlated (Table 5). However, the combined r2 value was only 7.0%.
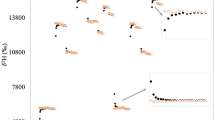
The relationships between age, [horizontal bar over]R, and mean dilution space expressed as a percentage of body weight are shown in Figures 1 and 2 for No and Nd, respectively. Both [horizontal bar over]R and percentage dilution space change with age, and these changes show a similar pattern for both isotopes.
In 35 infants measured at 6 wk and 6 mo, the mean change in R was-0.013 ± 0.024, significantly different from zero by paired t test (t = 3.13; p < 0.005).
DISCUSSION
Variation in the Nd:No ratio is consistently reported in the DLW literature. Increasingly, reports have emphasized that much if not all of this variation can be attributed to analytical error(4, 5), some of which may be related to choice of analytical methodology(16). Preterm infants are the only subjects in whom physiology has been thought to influence R significantly and consistently(16), and it has proved difficult to demonstrate a relationship between R and aspects of physiology such as body composition, age, or sex(4). Our analysis indicates that physiology does influence R in infants, and that after the magnitude of the effect is negligible compared with that of analytical error, different values of [horizontal bar over]R may be appropriate at different time points within the first year of life.
Our mean value of [horizontal bar over]R for the entire sample is very similar to that reported for adults, and our results confirm the previous report(10) that R approaches the adult value at a very young age. The lower values reported for preterm infants do not therefore appear to be applicable in general to normal healthy infants from 6 wk of age.
Nevertheless, previous reports have suggested that some variation in R may be physiologically derived(4), and after studies of preterm infants it was suggested that certain characteristics of young infants, including high body water content and rapid energy deposition, may influence R(8). Our study therefore investigated the relationship between physiology and R at a single time point, and between physiology and [horizontal bar over]R over the infant period.
At 12 wk of age, we found no relationship between R and percentage of body water, nor was R related to measures of s.c. adiposity, in keeping with previous reports(4, 17). Percentage body water is substantially higher in preterm infants than in healthy term infants(18), and tissue composition may also be different. We also found no difference in R between the sexes, whereas previous considerations of this issue in adults and children have shown conflicting results(4, 17). In contrast, weight gain was very weakly related to R, and this relationship may be due to the deposition of tissue during growth in early life, as suggested previously(8) and discussed further below. The effect of weight gain on R was negligible, explaining only 2.9% of the variation in R, and for practical purposes all between-subject variation at this time point can be assumed to be analytically derived. Use of a fixed ratio would therefore be an acceptable approach to calculating carbon dioxide production in these infants. However, further analysis indicates that the appropriate value of [horizontal bar over]R changes during the infant period.
Within the entire sample, correlation analyses indicated that sex, percentage of body water, and skinfold thicknesses were not related to R, whereas age and weight gain were related. Multiple regression analysis indicated that R fell with increasing age and with decreasing weight gain. However, a more distinct pattern was shown by considering the relationship between [horizontal bar over]R and age.Figures 1 and 2 show V-shaped curves for this relationship, which are very similar to the observed changes in mean percentage of body water over the same period. Moreover, the close relationship between [horizontal bar over]R and dilution space was apparent for both isotopes. This suggests that the change in [horizontal bar over]R is not due to changes in the proportion of deuterium, which exchanges with nonaqueous material, but rather is due to changes in the amount of nonaqueous material available for exchange. Thus the low value at 6 mo may be attributed to the low percentage body water, and hence high body fatness, at this time point, allowing less exchange of tracer with protein as explained below.
The overestimation of TBW by 2H and 18O occurs because of the exchange of the tracers with nonaqueous hydrogen and oxygen, and the difference in the extent of this overestimation occurs because more nonaqueous hydrogen than oxygen is available for exchange(2). Theoretical calculations indicate that most hydrogen exchange is with protein rather than fat(10), and our data are consistent with this theory in showing an association between percentage body water and [horizontal bar over]R. When percentage of body water is low and body fatness is high, there is relatively less protein available for exchange with deuterium, and [horizontal bar over]R is reduced.
The deposition of energy in growth is also predicted to influence R, due to the incorporation of tracer into fat and protein being synthesized(8). Again, the effect is greater for the hydrogen than the oxygen tracer, but unlike the exchange with deposited tissue, tracer incorporation occurs more in fat than in protein synthesis(19). Thus greater weight gain is predicted to increase R through increased incorporation of deuterium in fat synthesis, and the effect will be strongest in early life when the rate of fat deposition is relatively high(20). Again, our data are consistent with this prediction, in showing a positive relationship between R and weight gain. However, the magnitude of the effect is very low, and explains a negligible proportion of the between-subject variability in R. Even in rapidly growing infants, depositing up to 13 g of fat/d(20), the quantity of tracer incorporated into new tissue is likely to be too small to significantly influence calculation of carbon dioxide production.
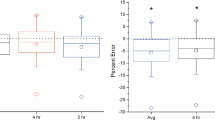
Use of an appropriate value for [horizontal bar over]R is important for accurate calculation of carbon dioxide production.Figure 3 shows the effect of R on estimated total energy expenditure, for a hypothetical correct value for [horizontal bar over]R of 1.030. If appropriate values for [horizontal bar over]R are as reported in the present study, use of the value 1.034 recommended for adults(4, 6) would lead to a mean overestimation in total energy expenditure of approximately 7% at 6 mo, and a mean underestimation of approximately 4% at 6 wk of age. These discrepancies would take on added importance if comparing infants of different ages.
Previous analyses of the most appropriate approach to R in infants have shown disagreement. In a validation study comparing DLW and energy balance methods, use of a fixed ratio in calculating total energy expenditure in infants at 1 and 4 mo of age underestimated energy expenditure by an average of 4%, whereas an approach using individual dilution spaces showed closer than 1% agreement(21). However, the two approaches differed with respect not only to R but also to other assumptions, for example whether insensible water loss was measured or estimated, which may also have influenced agreement with the balance method. Furthermore, both approaches showed a wide degree of interindividual variation in the extent to which isotopic and balance methods agreed. In contrast, a separate validation study showed good agreement between indirect calorimetry and DLW using a fixed ratio(22).
In conclusion, we have considered first whether use of a fixed ratio is acceptable in DLW studies of infants, and second if the appropriate value of [horizontal bar over]R is the same as that recommended for adults. We found no evidence that physiology exerts a significant effect on R in infants at a single time point, whereas analytical error appears to explain almost all observed variation between individuals. This indicates that use of a fixed ratio is an acceptable approach to calculating carbon dioxide production in a sample of homologous age. However, if a fixed ratio approach is used, it is necessary to use an appropriate value for [horizontal bar over]R. Our analysis suggests that [horizontal bar over]R changes with age during infancy, and this variation is associated with changes in percentage of body water, and by inference in body fatness. These changes in [horizontal bar over]R are large enough to have significant influence on calculation of energy expenditure. We therefore suggest that the values for [horizontal bar over]R reported here may be more suitable for use in infants than the value of 1.034 recommended for adults.
Abbreviations
- TBW:
-
total body water
- R:
-
ratio of 2H to 18O dilution space
- [horizontal bar over]R:
-
mean population value of R
- DLW:
-
doubly labeled water
- δ:
-
delta per million
- N:
-
dilution space
- Nd:
-
2H dilution space
- No:
-
18O dilution space
- SMOW:
-
standard mean ocean water
- Rs:
-
ratio of heavy:light molecules in sample
- Rsample:
-
ratio of heavy:light molecules in SMOW
References
Hevesy G, Hofer E 1934 Elimination of water from the human body. Nature 134: 879
Culebras JM, Moore FD 1977 Total body water and the exchangeable hydrogen. I. Theoretical calculation of non-aqueous exchangeable hydrogen in man. Am J Physiol 232:R54–R59
Sheng H-P, Huggins RA 1979 A review of body composition studies with emphasis on total body water and fat. Am J Clin Nutr 32: 630–647
Racette SB, Schoeller DA, Luke AH, Shay K, Hnilicka J, Kushner RF 1994 Relative dilution spaces of 2H and 18O-labeled water in humans. Am J Physiol 267:E585–E590
Speakman JR, Nair SK, Goran MI 1993 Revised equations for calculating CO2 production from doubly labeled water in humans. Am J Physiol 264:E912–E917
Coward WA, Ritz P, Cole TJ 1994 Revision of calculations in the doubly-labeled water method for measurement of energy expenditure in humans. Am J Physiol 267:E805–E807
Jensen CL, Wong WW, Butte NF, Schanler RJ, Klein PD 1989 Deuterium and 18-oxygen dilution spaces determined in preterm infants from samples of plasma, saliva and urine. Pediatr Res 25: 292
Jensen CL, Butte NF, Wong WW, Moon JK 1992 Determining energy expenditure in preterm infants: comparison of2 H218O method and indirect calorimetry. Am J Physiol 263:R685–R692
Butte NF, Wong WW, Ferlic L, Smith EO, Klein PD, Garza C 1990 Energy expenditure and deposition of breast fed and formula fed infants during early infancy. Pediatr Res 28: 631–640
Coward WA 1990 Calculation of pool sizes and flux rates. In: Prentice AM (ed) The Doubly-Labelled Water Method for Measuring Energy Expenditure. International Atomic Energy Agency, Vienna, pp 48–68
Coward WA 1988 The doubly-labelled water(2H218O) method: principles and practice. Proc Nutr Soc 47: 209–218
Roberts SB, Lucas A 1985 Measurement of urinary constituents and output using disposable napkins. Arch Dis Child 60: 1021–1024
Barrie A, Coward WA 1985 A rapid analytical technique for the determination of energy expenditure by the doubly labelled water method. Biomed Mass Spectrom 12: 535–541
Davies PSW, Wells JCK 1994 Calculation of total body water in infancy. Eur J Clin Nutr 48: 490–495
Ritz P, Cole TJ, Couet C, Coward WA 1996 Precision of DLW energy expenditure measurements: contribution of natural abundance variations. Am J Physiol 270:E164–E169
Ritz P, Johnson PG, Coward WA 1994 Measurements of2 H and 18O in body water: analytical considerations and physiological implication. Br J Nutr 72: 3–12
Goran MI, Poehlman ET, Nair SK, Danforth E 1992 Effect of gender, body composition, and equilibration time on the2 H-to-18O dilution space ratio. Am J Physiol 263:E1119–E1124
Wilson DC, Baird T, Scrimgeour CM, Halliday HL, Ried M, McClure G, Rennie MJ 1993 Total body water measurement by bioelectrical impedance in the extremely low birth weight infant. In: Ellis KJ, Eastman JD(eds) Human Body Composition: In Vivo Methods, Models and Assessments. Plenum, New York, pp 185–188
Haggarty P 1990 The effect of isotope sequestration and exchange. In: Prentice AM (ed) The Doubly-Labelled Water Method for Measuring Energy Expenditure. International Atomic Energy Agency, Vienna, pp 114–146
Fomon SJ, Haschke F, Ziegler EE, Nelson SE 1982 Body composition of reference children from birth to age 10 years. Am J Clin Nutr 35: 1169–1175
Wong WW, Butte NF, Garza C, Klein PD 1990 Comparison of energy expenditure estimated in healthy infants using the doubly labelled water and energy balance methods. Eur J Clin Nutr 44: 175–184
Jones PJH, Winthrop AL, Schoeller DA, Swyer PR, Smith J, Filler RM, Heim T 1987 Validation of doubly labeled water for assessing energy expenditure in infants. Pediatr Res 21: 242–246
Author information
Authors and Affiliations
Additional information
Supported in part by Ross Laboratories, Columbus, Ohio, and the Nestlé Foundation, Lausanne, Switzerland.
Rights and permissions
About this article
Cite this article
Wells, J., Ritz, P., Davies, P. et al. Factors Affecting the 2H to 18O Dilution Space Ratio in Infants. Pediatr Res 43, 467–471 (1998). https://doi.org/10.1203/00006450-199804000-00005
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199804000-00005
This article is cited by
-
Body composition reference charts for UK infants and children aged 6 weeks to 5 years based on measurement of total body water by isotope dilution
European Journal of Clinical Nutrition (2020)
-
Indices of whole-body and central adiposity for evaluating the metabolic load of obesity
International Journal of Obesity (2005)
-
Longitudinal investigation of energy expenditure in infants with cystic fibrosis
European Journal of Clinical Nutrition (2002)