Abstract
After anoxia-induced apnea, gasping remains the last operative mechanism for survival. In developing rats, the gasping response to anoxia exhibits triphasic characteristics. Because anoxia is associated with enhanced release of glutamate, we hypothesized that N-methyl-D-aspartate (NMDA) glutamate receptors may underlie components of the gasping response. Rat pups aged 2 d (n = 50), 5 d (n = 43), 10 d (n = 42), and 15 d (n = 45) underwent anoxic challenges with 100% N2 in a whole body plethysmograph, 30 min after intraperitoneal administration of MK801[(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d-]cyclohepten-5,10-imine hydrogen maleate; dizocilpine] (3 mg/kg), a noncompetitive NMDA glutamate receptor channel antagonist, or normal saline. In control pups, after primary apnea onset, a triphasic gasping pattern was apparent at all postnatal ages and included two distinct types of gasps (I and II). In 2- and 5-d MK801-treated animals, phase 1 and type I gasps were absent, leading to marked prolongations of the gasp latency and phase 2, the latter displaying type II gasps only. In addition, phase 3 duration was also prolonged with increased type II gasp frequencies. In contrast, in some 10-d-old (40%) and in all 15-d-old MK801-treated pups, although overall gasping duration was prolonged, the triphasic gasping pattern seen in matched controls was also present. We conclude that NMDA glutamate receptors mediate particular phasic components of the gasping response during early postnatal life but not at later stages of development. We speculate that developmental changes occur in both function and expression of NMDA and other neurotransmitters within brainstem regions underlying the neural substrate for gasp generation.
Similar content being viewed by others
Main
In mammals, exposure to asphyxic or anoxic conditions will elicit a characteristic pattern of respiratory responses. The initial hyperpnea that occurs upon administration of an anoxic gas is followed by primary apnea. Within a variable period of time (gasp latency), gasping respiratory efforts emerge and will finally cease (terminal apnea)(1). In the developing rat, the gasping period is not a homogeneous monophasic period and instead displays three recognizable phases (P1-P3) and two types of gasps(2). Type I gasps consist of an inspiratory effort that is preceded and followed by expiratory excursions(2). Type II gasps are devoid of the initial expiratory effort seen in type I gasps. The first phase (P1) consists of frequent type I gasps, whereas P2 is a relatively quiescent period in which type I or type II gasps occur a frequencies <2 gasps·min-1. Finally, P3 may be recognized by the onset of frequent type II gasps of progressively diminishing amplitude until all gasping activity ceases, i.e. terminal apnea(2). In addition to the overall increased tolerance to anoxia exhibited by younger rat pups(3, 4), significant age dependency was also identified in both the duration of each phase and the corresponding gasping frequencies, such that at 25 d postnatally, the triphasic gasping pattern had virtually disappeared and was replaced by the adult gasping response(2).
In adult and neonatal animals, the neural substrate underlying gasp formation has been ascribed to a discrete brainstem region, the LTF, located in a region that extends from dorsomedial to ventrolateral to the nucleus ambiguus in both cats and rats(5–8). Although the neurotransmitter properties of LTF neurons are currently unknown, physiologic recordings of the phrenic neurogram in adult cats during hypoxia before and after NMDA and non-NMDA receptor blockade do not support an important role for ionotropic glutamergic receptors in gasp generation(9). Similarly, the generation and transmission of eupneic respiratory drive in an in vitro brainstem neonatal rat preparation assigns important rhythmogenic properties to non-NMDA receptors rather than to NMDA receptors(10, 11). In a perfused brainstem preparation which preserves eupneic breathing, Hayashi and Lipski(12) showed that administration of antagonists of inhibitory amino acids resulted in the appearance of gasplike bursts. However, such limited evidence obtained mostly in adult mammals precludes identification of one or more ontogenetically regulated excitatory amino acid mechanisms potentially responsible for gasp neurogenesis. Indeed, anoxia elicits early neuronal depolarization with massive glutamate release to the extracellular space, and the subsequent activation of NMDA receptors increases intracellular calcium concentration and triggers a long lasting potentiation at the postsynaptic level(13). Thus, it is possible that NMDA receptors modulate important early phasic components of anoxia-induced gasping. Furthermore, brainstem regions exhibit marked postnatal changes in NMDA receptor subunit expression, suggesting maturational alterations in the neurotransmitter-receptor affinity and in their functional properties(14). In this study, we hypothesized that NMDA receptor blockade would alter the early phasic characteristics of anoxia-induced gasping in neonatal rats, and that such effect, if present, would gradually subside with postnatal maturation.
METHODS
The experimental protocols were approved by the Institutional Animal Use and Care Committee. Timed-pregnant Sprague-Dawley female rats were purchased(Charles River), and litter delivery times were recorded. Rat pups underwent anoxic challenges at 2 d (n = 50), 5 d (n = 43), 10 d(n = 42), and 15 d (n = 45) postnatally by sudden and sustained exposure to 100% nitrogen. Thirty minutes before the anoxic challenge, animals were randomly allocated to receive the noncompetitive NMDA channel antagonist MK801 (i.p., 3 mg·kg-1 dissolved in 0.2 mL of normal saline) or 0.2 mL of normal saline (control). Particular care was taken to ensure that a similar number of animals from the same litter would be allocated to MK801 and control groups.
Respiratory measures were continuously acquired in the unrestrained animal placed in a 500-mL plethysmographic chamber (Buxco Electronics, Troy, NJ), using the barometric method(15, 16). To minimize the effect of signal drift due to temperature and pressure changes outside the recording chamber, a reference chamber of equal size in which temperature was measured using a T-type thermocouple was used. To obtain respiratory signals, environmental temperature had to be maintained at 3-4°C below the thermoneutral range. Thus, in 2-10-d-old pups, environmental temperature was kept at ≈30°C, whereas in 15-d-old animals the environmental temperature was maintained at ≈28°C. Such precaution although dictated by constraints of the barometric technique had the additional advantage of providing a certain level of homeothermic standardization. Indeed, environmental temperature is of crucial importance to gasping duration(17, 18). A calibration volume of 0.5 mL of air was repeatedly introduced into the chamber before and upon completion of recordings. Humidified air or 100% N2 (90% relative humidity) was passed through at a rate of 8 L·min-1, using a precision flow pump-reservoir system. Transition from room air to anoxic conditions required approximately 30 s. Pressure changes in the chamber due to the inspiratory and expiratory temperature changes were measured using a high gain differential pressure transducer (Validyne, model MP45-1).
Analog signals were displayed on-screen, and continuously digitized and stored onto a Macintosh personal computer system at 125-Hz sampling frequency using MacLab Digital Acquisition Software (ADInstruments. Castle Hill, Australia) for subsequent off-line analysis. In addition, animals were under continuous visual inspection during the anoxic challenge, and observed events such as gasps and/or seizure activity were recorded in the digital record by one of the investigators.
Data are shown as mean ± SD. Digital records were partitioned in 1-min bins for analysis. The variables quantitated across postnatal ages included the number of gasps (types I and II), duration of gasps (time from primary apnea onset to terminal apnea), and gasping frequency. In addition, gasp latency was designated as the time from primary apnea to the first gasp. Type I gasps were defined as periodic respiratory excursions occurring after the onset of primary apnea and consisting of an initial expiratory effort (seeFig. 1). Type II gasps were defined as respiratory-related displacements consisting of an initial inspiratory effort. Differences between MK801-treated and control animals were assessed with two-tailed unpaired t tests. Comparisons across the various postnatal age groups were assessed two-way ANOVA and the Newman-Keuls multiple range test for multiple comparisons. A p value of <0.05 was considered to achieve statistical significance.
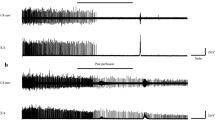
Illustrative physiologic recordings of respiratory signal during anoxia-induced gasping in a 2-d-old control rat pup(upper panel) and a littermate treated with MK801 (lower panel). The hyperpneic phase is indicated as well as the three recognizable gasping phases (1-3). Terminal apnea (cessation of measurable respiratory efforts) is shown by the arrow. Examples of type I and type II gasps are provided in the inset boxes.
RESULTS
Typical physiologic recordings of anoxia-induced gasping in a control and a MK801-treated 2-d-old rat pup are shown in Figure 1. These recordings demonstrate that type I gasps were virtually abolished by MK801 in young pups. The overall distribution of gasps over time is shown inFigure 2, and an age-dependent effect of MK801 on the pattern of gasps was apparent.
(A) Mean (± SD) number of gasps in 1-min bins of control rat pups at various postnatal ages. Time 0 represents occurrence of first gasp. (B) Mean (± SD) number of gasps in 1-min bins of MK801-treated rat pups at various postnatal ages. For comparison purposes, time 0 represents the average time at which the first gasp occurred in the age-matched control group.
MK801 significantly delayed the onset of the first gasp and prolonged the overall duration of gasping at all postnatal ages(Fig. 3; Table 1), an effect which did not display age dependency (2-way ANOVA, p = NS). The duration of the hyper-pneic phase preceding the onset of primary apnea was not affected by MK801 treatment (Table 1).
In 2- and 5-d-old pups, type I gasps were abolished by MK801 treatment(Fig. 4). In 10-d-old animals, MK801 reduced the overall number of type I gasps and in contrast, a significant increase in type I gasps occurred in 15-d-old animals (Fig. 4). Thus, significant reductions in type I gasping frequency resulted from MK801 treatment in the younger animals, whereas no changes in type I gasping frequency were observed in the 15-d-old group.
Mean (± SD) number of type I (left upper panel) and type II (left lower panel) gasps and corresponding gasping frequencies (right panels) in 2-, 5, 10-, and 15-d-old rat pups undergoing anoxic exposures after treatment with MK801 (hatched columns) or control (filled columns) (*p < 0.001; #p < 0.04).
In contrast with type I gasps, MK801 significantly increased the overall number of type II gasps across all postnatal ages (Fig. 4). However, type II gasping frequencies were significantly increased in 2- and 5-d-old pups only (p < 0.01), whereas in the older animals they remained unaltered (p = NS).
DISCUSSION
This study demonstrates that NMDA receptor blockade exerts profound changes in the early phases of anoxia-induced gasping in developing rat pups, and that these effects are age-dependent. Administration of MK801 was associated with significant prolongation of overall gasping duration, which was independent of the level of postnatal maturity. However, elimination of type I gasps and increases in type II gasping frequencies occurred with MK801 only in the younger animals, suggesting that, during this period of development, important changes in the role of NMDA emerge in relation to the neurogenesis of gasping.
The characteristic decrease in anoxic tolerance with increased maturation in mammals was present in both the MK801 and control groups(17–19). The mechanisms underlying such neonatal tolerance to anoxia are not yet completely understood. Diminished anoxic depolarization in in vitro neonatal brainstem neurons and increases in depolarization magnitude to adult-like levels by 3-4 wk of age have been reported(20). Such development differences in anoxic depolarization during tissue hypoxia could be ascribed to increases in excitatory amino acid concentrations(21), to changes in ATP-dependent potassium channels, and/or to voltage-sensitive sodium channels, because all these elements have been shown to modulate neuronal responses to hypoxia and anoxia(22–24). In addition, the relative contribution of regulatory mechanisms underlying cellular energetics and maintenance of intracellular ATP levels emerges as an additional important facet of neonatal tolerance to anoxia(25–28). In general, younger animals will decrease their metabolic requirements more efficiently during hypoxia, thus allowing for improved preservation of ATP stores. Current study design does not allow for elucidation of the relative importance of the above mentioned anoxic tolerance factors.
The increase in the duration of the gasping stage at all postnatal ages that followed NMDA blockade could reflect a decrease in calcium-mediated excitotoxicity during anoxia resulting from intracellular calcium influxes elicited by NMDA channel opening(29, 30). Indeed, prolongation of early gasping phases also occurred when animals were pretreated with a competitive NO synthase inhibitor(31), suggesting that similar to other neural structures, the NMDA-NO pathway plays an important role during the early stages of gasping(32, 33).
Of all the adaptive processes used by neonatal mammals to sustain life during anoxia or asphyxia, gasping emerges as the last operative mechanism for survival. Strains demonstrating robust and prolonged gasping efforts will be more likely to autoresuscitate when air or oxygen becomes available(34, 35). As originally proposed by Lumsden(36), gasping could represent a very primitive and resilient neuronal respiratory network that persisted through evolution and whose only function would be autoresuscitation. The existence of two types of barometrically recognizable gasps suggests the possibility that the neural gasping center or LTF(37) could encompass heterogeneous neuronal populations that undergo marked developmental alterations over time. An alternative and probably more likely explanation to these two types of gasps could be that type I gasps may not originate from the gasping center, but rather may represent a last burst of respiratory activity from the eupneic center(10, 11). Indeed, gasping activity appears to encompass inspiratory efforts only, with no evidence of expiratory activity being present during gasping(37). Irrespective of the source for neural activity underlying what we have termed here type I and type II gasps, our current findings suggest a potential speculative model whereby neurons mediating type I gasps are activated via NMDA receptors(Fig. 5). We further suggest that these NMDA neurons are either less tolerant to anoxia in the developing rat, or that their activity during anoxia will abate over time until their complete cessation. The latter could be the result of excitotoxic damage or reflect increases in GABA concentrations, which could gradually inhibit the discharge rates of these neurons (Figs. 1 and5). In the conceptual framework that we are proposing, another population of neurons that is more tolerant to anoxia would then become activated and generate the type II gasps(Fig. 5). The mechanisms underlying activation of this second population of LTF neurons are unclear. Regional GABA concentrations in the brain will increase with prolonged anoxia, and the magnitude of such GABA increase depends on the availability of α-ketoglutarate, because this compound is important in the degradation of GABA and synthesis of glutamate. During sustained or severe hypoxia, the level of α-ketoglutarate declines rapidly because the Krebs cycle intermediates depend on aerobic metabolism. With a reduction in α-ketoglutarate, GABA cannot be degraded, but it can still be formed from glutamate because GAD, the enzyme necessary for conversion of glutamate to GABA, is an anaerobic enzyme, and therefore allows for GABA formation to proceed during hypoxia(38, 39). Thus, increasing GABA formation during prolonged anoxia could increasingly recruit type II gasp neurons, and modulate their discharge pattern until all residual energetic stores available would become exhausted, i.e. terminal apnea. In this setting, GABA could function as an excitatory rather than inhibitory postsynaptic mediator, especially during early postnatal life(40). In support of such a hypothetical scheme, type II gasp frequencies were enhanced only in the younger pups, whereas no changes occurred in older animals. However, our proposed scheme does not provide insights into what other neurotransmitters may contribute to gasping neurogenesis. More recent work describing the connectivity and regional projections of neurons within the LTF is an important initial step in such direction(41).
Schematic diagram of hypothetical mechanisms potentially underlying anoxia-induced gasping during the early phases of postnatal life. Anoxia will lead to glutamate (Glu) release which will initially activate neurons mediating type I gasps via NMDA receptors. Such neurons may express NO synthase, and resultant NO production may serve as a retrograde messenger for further glutamate release. GABA will also be generated over time by ongoing GAD, and type I neuronal activity will cease with onset of anoxic depolarization. At this point in time, neurons with a high degree of anoxic tolerance and expressing GABA receptors will become activated and mediate type II gasp generation.
The developmental patterns of NMDA, GABA receptor, and GAD expression in the LTF are currently unknown. The pharmacologic and physiologic properties of ligand-gated ion channels are dependent on their subunit composition, and spontaneously occurring changes in subunit composition during neuronal development may result in dramatic functional differences between embryonic and adult forms of the receptor complex. Luo et al.(42) have recently demonstrated that the NR1 subunit expression of NMDA receptor undergoes a marked increase spanning over the first 3 wk of postnatal life in several brain regions including the brainstem. However, this increase may be heterotopically distributed, suggesting that the functional and pharmacologic diversity in the NMDA receptor properties is continuously evolving postnatally(14). Similarly, significant maturation in GABAergic systems and in GABA receptor expression occurs during the first 2 wk of life(43, 44). Finally, a significant increase in GAD activity occurs in the rat brain, more particularly during the 2nd wk of life(45), suggesting an increased ability to generate GABA with maturation. All of the above maturational changes in the glutamate and GABA pathways could lead to substantial alterations in recruitment patterns and functional properties of neurons mediating the gasping response.
In summary, we demonstrate that NMDA receptor blockade prolongs the gasping phase duration in rat pups irrespective of their degree of maturation, and that NMDA receptors mediate early gasp generation during anoxic conditions in young rat pups. However, such process is replaced later in postnatal life by mechanisms that remain to be defined.
Abbreviations
- GABA:
-
γ-aminobutyric acid
- GAD:
-
glutamic acid decarboxylase
- LTF:
-
lateral tegmental field of the medulla
- MK801 :
-
(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate dizocilpine
- NMDA:
-
N-methyl-D-aspartate glutamate
- NO:
-
nitric oxide
References
Campbell AGM, Cross KW, Dawes GS, Hyman AI 1966; A comparison of air and oxygen in a hyperbaric chamber or by positive pressure ventilation in the resuscitation of newborn rabbits. J Pediatr 68: 153–163.
Gozal D, Torres JE, Gozal YM, Nuckton TJ 1996; Characterization and developmental aspects of anoxia-induced gasping. Biol Neonate 70: 280–288.
Stafford A, Weatherall JAC 1960; The survival of young rats in nitrogen. J Physiol 153: 457–472.
Duffy TE, Kohle SJ, Vanucci RC 1975; Carbohydrate and energy metabolism in perinatal rat brain: relation to survival in anoxia. J Neurochem 24: 271–276.
St. John. WM, Bledsoe TA, Sokol HW 1984; Identification of medullary loci critical for neurogenesis of gasping. J Appl Physiol 56: 1008–1019.
Lumsden T 1923; Observations on the respiratory centres in the cat. J Physiol 57: 153–160.
Fung ML, Wang W, St. John WM 1994; Medullary loci critical for expression of gasping in adult rats. J Physiol 488: 597–611.
Wang W, Fung ML, Darnall RA, St. John WM 1996; Characterizations and comparisons of eupnoea and gasping in neonatal rats. J Physiol 490: 277–292.
Chae LO, Melton JE, Neubauer JA, Edelman NH 1993; Phrenic and sympathetic nerve responses to glutamergic blockade during normoxia and hypoxia. J Appl Physiol 74: 1954–1963.
Greer JJ, Smith JC, Feldman JL 1991; Role of excitatory amino acids in the generation and transmission of respiratory drive in neonatal rat. J Physiol 437: 727–749.
Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL 1991; Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254: 726–729.
Hayashi F, Lipski J 1992; The role of inhibitory amino acids in control of respiratory motor output in an arterially perfused rat. Respir Physiol 89: 47–63.
Szatowski M, Attwell D 1994; Triggering and execution of neuronal death in brain ischemia: two phases of glutamate release by different mechanisms. Trends Neurosci 17: 359–365.
Benke D, Wenzel A, Scheuer L, Fritschy JM, Mohler H 1995; Immunobiochemical characterization of the NMDA-receptor subunit NR1 in the developing and adult rat brain. J Recept Signal Transduct Res 15: 393–411.
Bartlett DJB, Tenney SW 1970; Control of breathing in experimental anemia. Respir Physiol 10: 384–395.
Pappenheimer JR 1977; Sleep and respiration of rats during hypoxia. J Physiol 266: 191–207.
Fazekas JF, Alexander FAD, Himwich HE 1941; Tolerance of the newborn to anoxia. Am J Physiol 134: 281–287.
Miller JA 1949; Factors of neonatal resistance to anoxia. I. Temperature and survival of guinea pigs under anoxia. Science 110: 113–114.
DeHaan RL, Field J. Roth E 1959; Anoxic endurance of cardiac and respiratory function in the adult and infant rat. Am J Physiol 197: 445–448.
Haddad GG, Donnelly D 1990; O2 deprivation induces a major depolarization in brainstem neurons in the adult but not in the neonatal rat. J Physiol 429: 411–428.
Liu Z, Stafstrom CE, Sarkisian M, Tandon P, Yang Y, Hoi A, Holmes GL 1996; Age-dependent effects of glutamate toxicity in the hippocampus. Dev Brain Res 97: 178–184.
Young RSK, During MJ, Donnelly DF, Aquila WJ, Perri VL, Haddad GG 1993; Effect of anoxia on excitatory amino acid in brain slices of rats and turtles: in vitro microdialysis. Am J Physiol 264:R716–R719.
Jiang C, Xia Y, Haddad GG 1992; Role of ATP-sensitive K+ channels during anoxia: major differences between rat (newborn and adult) and turtle neurons. J Physiol 448: 599–612.
Cummins TR, Jiang C, Haddad GG 1993; Human neocortical excitability is decreased during anoxia via sodium channel modulation. J Clin Invest 91: 608–615.
Dawes GS, Mott JC, Shelley HJ 1959; The importance of cardiac glycogen for the maintenance of life in foetal lambs and newborn animals during anoxia. J Physiol 146: 516–538.
Lowry OH, Passoneau JV, Hasselberger FX, Schulz DW 1964; Effect of ischemia on known substrates and cofactors of the glycolytic pathways in brain. J Biol Chem 239: 18–30.
Haddad GG, Jiang C 1993; O2 deprivation in the central nervous system: on mechanisms of neuronal response differential sensitivity and injury. Prog Neurobiol 40: 277–318.
Kass IS, Lipton P 1989; Protection of hippocampal slices from young rats against anoxic transmission damage is due to better maintenance of ATP. J Physiol 413: 1–11.
Zanelli S, Numagami Y, Mishra OP, Delivoria-Papadopoulos M 1996; Mechanisms of calcium influx in cortical synaptosomes of the guinea pig fetus. Pediatr Res 40:A257.
Zanelli S, Numagami Y, Mishra OP, Delivoria-Papadopoulos M 1996; Synaptosomal calcium influx in the cortex of the hypoxic guinea pig fetus. Pediatr Res 40:A258.
Gozal D, Torres JE, Gozal YM, Nuckton TJ 1996; Nitric oxide (NO·) modulates the anoxic gasping response in developing rats. Am J Respir Crit Care Med 153:A80.
Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH 1991; Nitric oxide mediates glutamate neurotoxicity in primary cortical culture. Proc Natl Acad Sci USA 88: 6368–6371.
Lawrence AJ, Jarrott B 1993; Nitric oxide increases interstitial excitatory amino acid release in the rat dorsomedial medulla oblongata. Neurosci Lett 151: 126–129.
Gershan WM, Jacobi MS, Thach BT 1990; Maturation of cardiorespiratory interactions in spontaneous recovery from hypoxic apnea(autoresuscitation). Pediatr Res 28: 87–93.
Jacobi MS, Thach BT 1989; Effect of maturation on spontaneous recovery from hypoxic apnea by gasping. J Appl Physiol 66: 2384–2390.
Lumsden T 1923; Observations on the respiratory centres. J Physiol 57: 354–367.
St John WM 1996; Medullary regions for neurogenesis of gasping: noeud vital or noeuds vitals? J Appl Physiol 81: 1865–1877.
McGeer PL, McGeer EG 1981; Amino acid neurotransmitters. In: Siegel CJ, Albers RW, Katzman R, Agranoff BW (eds) Basic Neurochemistry, Little Brown, Boston. 233– 252.
Martin DL, Rimwall K 1993; Regulation ofγ-aminobutyric acid synthesis in the brain. J Neurochem 60: 395–407.
Gaiarsa JL, Corradetti R, Cherubini E, Ben-Ari Y 1990; The allosteric glycine site of the N-methyl-D-aspartate receptor modulates GABAergic-mediated synaptic events in neonatal rat CA3 hippocampal neurons. Proc Natl Acad Sci USA 87: 343–346.
Fung ML, Huang Q, Zhou D, St John WM 1997; The morphology and connections of neurons in the gasping center of adult rats. Neuroscience 76: 1237–1244.
Luo J, Bozy TZ, Wang Y, Yasuda RP, Wolfe BR 1996; Ontogeny of NMDA R1 subunit protein expression in five regions of rat brain. Dev Brain Res 92: 10–17.
Gaiarsa JL, McLean H, Congar P, Leinekugel X, Khazipov R, Tseeb V, Ben-Ari Y 1995; Postnatal maturation of γ-aminobutyric acid A and B-mediated inhibition in the CA3 hippocampal region of the rat. J Neurobiol 26: 339–349.
Chang CC, Luntz-Leybman V, Evans JE, Rotter A, Frostholm A 1995; Developmental changes in the expression ofγ-aminobutyric acid A/benzodiazepine receptor subunit mRNAs in the murine inferior olivary complex. J Comp Neurol 356: 615–628.
Balcar VR, Zetzsche T, Wolff JR 1992; Glutamate decarboxylase in developing rat neocortex: does it correlate with the differentiation of GABAergic neurons and synapses? Neurochem Res 17: 253–260.
Author information
Authors and Affiliations
Additional information
Supported in part by a grant from the National Institute of Child Health and Development, HD-01072, and by a Bureau of Maternal and Child Health Training Grant MCJ-229163. D.G. is the recipient of a Career Development Award from the American Lung Association.
Rights and permissions
About this article
Cite this article
Gozal, D., Torres, J. Maturation of Anoxia-Induced Gasping in the Rat: Potential Role forN-Methyl-D-Aspartate Glutamate Receptors. Pediatr Res 42, 872–877 (1997). https://doi.org/10.1203/00006450-199712000-00025
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199712000-00025