Abstract
Sudden unexpected death in epilepsy (SUDEP) is the leading epilepsy-related cause of premature mortality in people with intractable epilepsy, who are 27 times more likely to die than the general population. Impairment of the central control of breathing following a seizure has been identified as a putative cause of death, but the mechanisms underlying this seizure-induced breathing failure are largely unknown. Our laboratory has advanced a vascular theory of postictal behavioural dysfunction, including SUDEP. We have recently reported that seizure-induced death occurs after seizures invade brainstem breathing centres which then leads to local hypoxia causing breathing failure and death. Here we investigated the effects of caffeine and two adenosine receptors in two models of seizure-induced death. We recorded local oxygen levels in brainstem breathing centres as well as time to cessation of breathing and cardiac activity relative to seizure activity. The administration of the non-selective A1/A2A antagonist caffeine or the selective A1 agonist N6-cyclopentyladenosine reveals a detrimental effect on postictal hypoxia, providing support for caffeine modulating cerebral vasculature leading to brainstem hypoxia and cessation of breathing. Conversely, A2A activation with CGS-21680 was found to increase the lifespan of mice in both our models of seizure-induced death.
Similar content being viewed by others
Introduction
Sudden unexpected death in epilepsy (SUDEP) is one of the most common causes of death associated with epilepsy. SUDEP accounts for 17% of all epilepsy-related deaths and nearly 50% of deaths as a direct result of epilepsy in individuals with chronic refractory epilepsy1,2,3. The standardized mortality rate in young adults (20–40 years) makes SUDEP the second leading neurological cause of death in terms of years of potential life lost, behind stroke4,5,6,7. Several mechanisms have been proposed for SUDEP including cardiac, arousal and neurotransmitter dysfunction and obstructive apneas8,9,10. Although the underlying mechanism remains elusive, data from epilepsy monitoring units have refined our understanding of SUDEP and provide evidence that SUDEP is ultimately caused by a failure to breathe soon after a seizure ends11.
While the pathophysiological underpinnings of postictal respiratory failure remains elusive, emerging data highlight a candidate cause12,13,14,15,16,17. In 2016, Farrell and colleagues demonstrated that seizures in the forebrain induce postictal vasoconstriction leading to hypoperfusion and brain tissue hypoxia in both rodents and humans and is severe enough to cause behavioural dysfunction12,16. George and colleagues (2023) recently tested the neurovascular theory of SUDEP and determined that localized brainstem postictal hypoxia was a key driver in seizure-induced mortality in both acute and chronic mouse models, thus providing the mechanistic antecedent to the collapse of cardiorespiratory function which characterizes SUDEP17.
Further probing the neurovascular consequence of seizures, Phillips, and colleagues (2019) determined that acute caffeine and its metabolites reduced baseline hippocampal oxygen levels and worsened seizure-induced hypoxia18. Caffeine is the most widely consumed psychoactive drug in the world with an average intake around 300 mg/day19,20. Currently, limited caffeine consumption has been advised for people with epilepsy by some authors21,22,23. However, patient education on caffeine avoidance is not standard which may impart harm on those with epilepsy and at greatest risk for SUDEP20.
The present study tested the neurovascular theory of SUDEP and utilized pharmacological approaches to examine the effects of caffeine and A1 and A2A receptors on two mouse models of seizure-induced death while simultaneously recording brain tissue oxygen levels in the hippocampus and brainstem breathing centers. Acutely, using intrahippocampal kainic acid and chronically using Kcna1−/− mice, we found that caffeine significantly accelerated time to death in both models, indicating that caffeine consumption could be a modifiable risk factor in SUDEP pathophysiology.
Results
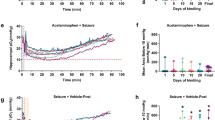
Acute caffeine, theophylline and N6 accelerate time to death whereas CGS-21680 delays it
Having previously established an acute model of seizure-induced death in mice14, we sought to screen caffeine and other drugs of interest to determine their relationship to SUDEP (Fig. 1). We first sought to determine if adenosine receptor modulation had an effect on time to seizure-induced death. Here we tested agonists and antagonists on the two primary adenosine receptors expressed in the brain: A1 and A2A. After intrahippocampal administration of the chemoconvulsant kainic acid in C57BL/6J mice, all mice were monitored using cardiorespiratory physiology and brain tissue oxygen to establish time to death defined as terminal apnea detected by diaphragmatic EMG17. All mice displayed several bouts of bilateral tonic–clonic seizures with intervening behavioral recovery. On average, mice that received vehicle died of seizure-induced terminal apnea 49.78 ± 1.30 min after intrahippocampal administration of kainic acid (Fig. 2). Mice that were treated with caffeine a non-selective A1/A2A antagonist died sooner than vehicle mice with a mean time to death of 27.21 ± 2.65 min. We then examined theophylline, a metabolite of caffeine and a non-selective A1/A2A antagonist. Theophylline displayed a similar temporal profile to caffeine, having their time to death accelerated with a mean time to death of 30.32 ± 3.67 min. To determine if caffeine was interacting with the A1 receptor to influence time to death, we used the selective A1 agonist N6-cyclopentyladenosine (N6). Mice that were treated with N6 had their time to death accelerated with a mean time to death of 35.95 ± 4.27 min. We next determined if caffeine’s influence on time to death was mediated by the A2A receptor. To this end we used CGS-21680, a selective A2A agonist. Mice that received CGS-21680 displayed increased survival compared to vehicle, caffeine and N6 treated mice with a mean time to death of 114.0 ± 5.72 min. Our data provide evidence that caffeine accelerated time to death likely independent of the A1 receptor as the use of the A1 agonist N6 mirrored the effects of caffeine, a non-selective antagonist. Using CGS-21680 as a probative tool, caffeine is likely interacting at the A2A receptor to accelerate time to death from hypoxia.
Acute experimental timeline. Male C57BL/6J mice on P50 underwent brain implantation surgery and diaphragmatic surgery (Day 0). Mice recovered for one week before beginning terminal experiment (Day 7). Mice were injected with either caffeine, theophylline, N6-Cyclopentyladenosine (N6) or CGS- 21,680, 30 min prior to infusion of kainic acid. Physiological parameters including heart rate, respiratory rate, brain partial pressure of oxygen and local field potential were recorded until death.
Drug targets of adenosine A1 and A2A receptor subtype. (A) Model schematic: following the injection of vehicle or drug, cardiorespiratory (HR: heart rate/ RR respiratory rate), oxygen and LFP signals were measured. electromyograph (EMG) electrode wires record respiratory activity directly from diaphragm and electrodes record cardiac activity. A bipolar electrode in the brainstem records local field potential (LFP) in the pre-Bötzinger complex (PBC) and an oxygen sensing optode in the contralateral PBC records absolute oxygen. Mean time to death in male C57BL6/J mice treated with drugs targeting adenosine receptor subtypes. (B) Vehicle mice (n = 11) mean time to death was 50.44 ± 3.95, Caffeine mice (n = 11) mean time to death was 27.21 ± 2.65 (t20 = 4.87, p = < 0.0001****). (C) Vehicle mice (n = 10) mean time to death was 51.80 ± 3.17, Theophylline (n = 10) mean time to death was 30.32 ± 3.67 (t18 = 4.42, p = 0.0003***). (D) Vehicle mice (n = 10) mean time to death was 49.12 ± 2.44, N6 (n = 10) mean time to death was 35.95 ± 4.27 (t18 = 2.67, p = 0.0154*). (E) Vehicle mice (n = 10) mean time to death was 19.12 ± 2.44, CGS-21680 (n = 10) 114.0 ± 5.72 (t18 = 10.42, p = < 0.0001****). Histobars represent mean ± SEM.
Acute caffeine reduces baseline brainstem oxygen
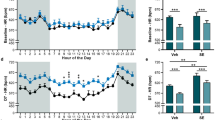
A previous study examining rat hippocampal oxygen levels demonstrated that caffeine significantly reduces baseline oxygen and exacerbated seizure induced hypoxia18. Here we monitored brain tissue oxygenation in the hippocampus (HPC) and the pre-Bötzinger complex (PBC), a respiratory nucleus located in the ventral medulla responsible for the generation of rhythmic inspiratory breathing movements in C57BL/6J mice24. We measured oxygen levels at three time points prior to intrahippocampal kainic acid infusion to examine the influence of adenosinergic agonists and antagonists on physiological oxygen values in the HPC and PBC of the brainstem (Fig. 3). We examined baseline oxygen levels at pre-injection of drug or vehicle (T-1), 10-min post-injection of drug or vehicle (T-2) and 30-min post-injection of drug or vehicle (T-3). Vehicle treated mice (n = 11) did not have significantly different oxygen values in the hippocampus or brainstem at any time point. Mice that were treated with caffeine (n = 11) displayed significant reductions between T-1 baseline and T-3 in the hippocampus showing a 38.15% difference in partial pressure of oxygen. Caffeine also significantly lowered oxygen values in the brainstem. There was a 31.33% difference between T-1 and T-2 and a 42.64% difference in oxygen between T-1 and T-3 baseline. Mice treated with the A2A agonist CGS-21680 (n = 11) had significant increase in HPC oxygen between T-1 and T-3 with a difference of 30.61%. In the PBC, there was a 21.80 difference between T-1 and T-3 and a 24.34% difference between T-2 and T-3. Our data indicate that caffeine, an A2A antagonist, decreases baseline oxygen values in the forebrain and brainstem. In opposition, CGS-21680, an agonist at the A2A receptor, raises baseline oxygen in both brain regions.
Caffeine reduced baseline brainstem oxygen. Mean partial pressure of oxygen (mmHg) pre-injection of drug or vehicle baseline (T-1); 10-min post-injection of drug or vehicle baseline (T-2); Mean 30-min post-injection of drug or vehicle baseline (T-3). (A,B) Hippocampus (HPC) and pre-Bötzinger complex (PBC) oxygen differences were not statistically significant in vehicle mice (n = 9). (C) HPC caffeine: (n = 9), T-1 = 23.51 ± 1.93, T-2 = 19.46 ± 1.72, T-3 = 15.95 ± 1.62, (ANOVA F (2, 24) = 4.567, p = 0.0209). (D) PBC caffeine: T-1 = 50.64 ± 2.72, T-2 = 36.93 ± 3.02, T-3 = 32.85 ± 1.43, (ANOVA F (2, 24) = 13.90, p = < 0.0001***). (E) HPC CGS-21680: (n = 9), T-1 = 25.68 ± 1.80, T-2 = 27.67 ± 2.84, T-3 = 34.96 ± 2.31, (ANOVA F (2, 24) = 4.281, p = 0.026). PBS CGS-21680: T-1 = 45.03 ± 2.62, T-2 = 39.18 ± 2.98, T-3 = 56.04 ± 2.02, (ANOVA F (2,24) = 11.09, p = 0.0004***). Histobars represent mean ± SEM.
Acute caffeine administration accelerates time to terminal hypoxia, apnea and death
We have demonstrated that caffeine significantly reduces baseline hippocampal and brainstem oxygen while CGS-21680 significantly increases its levels. Our next step was to establish the sequence of events that occur with cardiorespiratory function and oxygen following the administration of either caffeine or CGS-21680. Following the intrahippocampal administration of kainic acid in C57BL/6J mice, the partial pressure of oxygen (pO2) remained above 10 mmHg. Oxygen levels at or below this threshold are considered severely hypoxic and are associated poor clinical outcomes leading to increased morbidity and mortality25. Vehicle treated mice displayed the following sequence of events leading to death: terminal electrographic seizure in the PBC, with behavioural bilateral tonic–clonic seizure, terminal hypoxia in the HPC, followed terminal hypoxia in the PBC with concomitant terminal apnea occurring once pO2 dropped below 4.8 ± 1.84 mmHg and finally followed by asystole several minutes later. Mice that were treated with caffeine displayed the same sequence of events, however, it was temporally accelerated with apnea occurring once pO2 dropped bellow 5.1 ± 2.18 mmHg. Mice that were pretreated with CGS-21680, displayed significantly longer latency to time of death compared to vehicle and caffeine mice (Fig. 4).
Acute caffeine administration decreases time to terminal apnea hypoxia and death. Representative profile from three C57BL/6J + kainic acid mice. (A,Ai) The mean time to terminal hypoxia of the hippocampus (HPC) was 51.93 ± 2.47 in vehicle (n = 11), 26.22 ± 2.58 in caffeine (n = 11) and 94.45 ± 10.11 in CGS-21680 (n = 11) mice ANOVA F (2,30) = 30.98, p = < 0001***. (B,Bi) Mean time to terminal hypoxia of the pre-Bötzinger complex (PBC) was 52.32 ± 2.49 in vehicle, 26.37 ± 2.70 in caffeine and 101.2 ± 10.10 in CGS-21680 mice ANOVA F (2,30) = 37.46, p = < 0.001****. (C,Ci) Mean time to terminal apnea in was 52.80 ± 2.52 in vehicle, 27.21 ± 2.65 in caffeine and 103.4 ± 10.01 in CGS-21680 mice ANOVA F (2,30) = 39.67, p = < 0.0001****. (D,Di) Mean time to terminal asystole was 58.70 ± 2.69 in vehicle, 32.91 ± 2.61 in caffeine and 109.6 ± 9.92 in CGS-21680 mice ANOVA F (2,30) = 40.49, p = < 0.0001****. Box plots represent mean ± SEM.
Chronic adenosine receptor modulation influences time to death in Kcna1−/− mice
Our acute intrahippocampal kainic acid experiments indicated that a single dose of caffeine or its metabolite significantly accelerated time to death and CGS-21680 reduced time to death. Here we determined if the observations made in the acute experiments could be replicated chronically using Kcna1−/− mice which displays frequent self-generated seizures beginning in early life through to adulthood with high incidence of premature seizure-induced death26. On postnatal day (P) 30 mice were randomly assigned to either caffeine-in-water or water-only groups. The median survival time was significantly shorter in mice treated with caffeine (n = 9) compared to the water-only (n = 9) group (P37 vs. P42, respectively). Our acute experiments also indicated that a single dose of the A2A agonist, CGS-21680, significantly extended life. To test whether chronic CGS-21680 could also produce significant life extension, we administered this drug chronically. Due the solubility limitations, we administered CGS-21680 intracerebroventricularly via an osmotic minipump. The median survival time was significantly longer in mice treated with CGS-21680 (n = 9) compared to the water only (n = 9) group (P51 vs. P40 respectively) (Fig. 5). These data clearly demonstrate that chronic ingestion of caffeine may be detrimental in those with epilepsy, increasing the likelihood of premature mortality. Conversely, the A2A agonist CGS-21680 was shown to be neuroprotective.
Chronic caffeine accelerates time to death and chronic CGS-21680 delays death. (A,B) (A) Kcna1−/− mice treated with caffeine (n = 9) lived significantly shorter compared to vehicle (n = 9) treated mice. Median survival day for vehicle mice was 42d compared to median survival of caffeine treated of 37d p = 0.0015** (Log-rank test) (Ai). Mean survival day for vehicle treated mice was 41.89 ± 0.99 compared to mean survival of caffeine treated mice 37 ± 0.72, t16 = 3.97, p = 0.0011**. Chronic CGS-21680 reduces premature mortality and extends life, while chronic caffeine increases premature mortality. (B) Kcna1−/− mice treated with CGS-21680 (n = 9) lived significantly longer compared to vehicle (n = 9) treated mice. Median survival day for vehicle mice was 44d compared to median survival of CGS-21680 of 51d ****p = < 0.0001 (Log-rank test), (Bi) Mean survival day for vehicle treated mice was 44 ± 0.52 compared to mean survival of CGS-21680 treated mice 51.78 ± 1.05, t16 = 6.61, p = < 0.0001****. Histobars represent mean ± SEM.
Discussion
Self-limited seizures in both rodents and people with epilepsy result in vasoconstriction, hypoperfusion and hypoxia12,13,14. Postictal hypoxia was hypothesised to lead to SUDEP16. George and colleagues (2023) recently tested this neurovascular based hypothesis of SUDEP and found that seizure activity in brainstem breathing centres led to severe hypoxia and subsequent breathing failure17. They also identified two drug targets, cyclooxygenase-2 and L-type calcium channels, that when blocked prevented postictal hypoxia for a period of time and extended life in their models of seizure-induced death. Caffeine and other drugs that modulate adenosine receptors were previously found to increase the length of postictal hypoxia18. In the present study we observed that caffeine significantly lowers baseline oxygen in the hippocampus and brainstem and accelerates time to death. We provide evidence against caffeine’s involvement of the A1 receptor by using the selective A2A agonist CGS-21680 which was able to increase baseline oxygen and decrease time to death. Our study is the first to show an association between caffeine and postictal hypoxia in SUDEP pathophysiology.
We demonstrated that caffeine accelerated time to death in both our acute and chronic models of seizure-induced death. This study made the critical observation that acute dosing of caffeine significantly reduced baseline physiological oxygen in both the hippocampus and pre-Bötzinger complex. The neurovascular theory of SUDEP posits that seizures that spread to the brainstem lead to hypoxia of breathing centres and subsequent breathing failure. The pre-seizure reduction in oxygen seen in response to caffeine can serve to increase the likelihood of fatal outcomes as it sets the stage for death. The cerebral vasoconstrictive effects of caffeine are well established27,28,29. Caffeine exerts its effect as a vasoactive substance on a wide range of molecular targets30. Primarily, it acts as a non-selective antagonist of the A1 and A2A adenosine receptors. We first isolated the receptor subtype responsible for promoting caffeine’s effect on postictal hypoxia and early death in our models. We first examined A1 activation using the selective A1 agonist N6-Cyclopentyladenosine (N6). In our hands, N6 showed a similar effect to caffeine by accelerating time to death. We used the A2A selective agonist CGS-21680 which reduced time to death in both our acute and chronic models. Based on these receptor pharmacology results, we have evidence that caffeine is likely interacting with A2A receptors to produce its vascular effects.
Contrary to our findings, a previous study in mice showed that acute caffeine administration increased survival in a model of seizure-induced death31. In their study, to identify if increased adenosine was responsible for SUDEP, caffeine was administered after seizure onset following systemic kainic acid administration. Their study however did not quantify physiological parameters such as respiratory and cardiac physiology which limits the ability to draw conclusions regarding the cause of death. Further, the caffeine dose of 40 mg/kg exceeds the equivalent dose consumed by a typical human, reducing clinical translatability32.
The present study utilized two previously published models of seizure-induced death: acute intrahippocampal kainic acid which causes several bouts of bilateral tonic–clonic seizures with a resultant terminal seizure, and chronic Kcna1−/− mice that display self-generated bilateral tonic–clonic seizures early in life with a high incidence of seizure-induced death17. These models were chosen as they recapitulate core features of SUDEP in humans, including young age, high seizure burden, and bilateral tonic–clonic seizures. As a function of these all-or-none seizure-induced death models, all mice died regardless of treatment as these drugs were administered to assess how they modulate time to death. We recorded several physiological parameters to assess the sequence of events leading to death. Breathing in this study was measured using a diaphragmatic EMG which records phrenic nerve activity. The EMG method determines cessation of diaphragmatic contraction and does not measure ventilation. Therefore, we cannot rule out a potential peripheral obstruction such as a laryngospasm. This model also produces several bouts of recurrent seizures which can lead to metabolic derangements and contribute to premature death. In our chronic study, Kcna1−/− mice that received caffeine or CGS-21680 did not have their seizures recorded which limits our ability to assess whether latency to death was mediated by anti-seizure effects.
The intrahippocampal kainic acid model provides a unique tool to examine the physiological alterations that occur during and after a seizure, leading to mortality. High doses of kainic acid can evoke status epilepticus (SE)33 which precludes a diagnosis of SUDEP. However, lowering the dose can produce non-status models34,35. Previous reports have established cerebral oxygen markers of SE in rodent models. Using oxygen sensitive probes, it has been demonstrated that SE produces robust and long-lasting hyperoxia which correlates with increased cerebral blood flow36,37. This is also consistent with other reports that show sustained cerebral hyperperfusion following kainic acid-induced SE38 and pilocarpine- induced SE39,40. During recordings in this study, all mice had at least two behavioral seizures followed by postictal hypoxia and severe hypoxia prior to death, which is not associated with SE.
Brain function depends critically on moment-to-moment regulation of oxygen supply by the bloodstream to meet changing metabolic needs as seen during seizures. In fact, seizures significantly increase metabolic rate and oxygen consumption by mitochondria, severely limiting the availability of oxygen within the system41. This metabolic reduction in oxygen could contribute to seizure-induced mortality if it involved the brainstem and was temporally correlated to postictal vasoconstriction. Recently, Villa and colleagues (2023) demonstrated that postictal mitochondrial dysfunction and the unchecked conversion of oxygen to reactive oxygen species also contributes to postictal severe hypoxia42. Further they found that using a mild mitochondrial uncoupler which prevents the conversion of oxygen to reactive oxygen species ameliorated postictal hypoxia and provide a potential therapeutic treatment to reduce the risk of SUDEP42.
The endogenous nucleoside adenosine plays an important role in several physiological processes43. In the central nervous system, adenosine imparts an inhibitory tone on synaptic activity and is a potent modulator cerebral blood flow and tissue oxygenation43,44,45. Adenosine levels in the brain increase during pathological states such as ischemia, hypoxia, and seizures46,47 and may play a role in seizure termination. Despite its antiseizure properties, excessive adenosine is postulated to exert detrimental effects in the brainstem48,49. The adenosine hypothesis of SUDEP states that seizure-induced increase of adenosine in the brainstem leads to inhibition of breathing circuits via the activation of adenosine receptors31,48,49. However, contrary to this hypothesis, we show that activation of the A2A receptor is beneficial by extending life. The endogenous ligand for the A2A receptor is adenosine. Among the adenosine receptors, A2A is the primary receptor responsible for the vasodilatory effects of adenosine.
We also demonstrate that the A2A agonist CGS-21680 both increased baseline hippocampal and brainstem oxygen and prolonged time to death. Activation of the A2A receptor mediates vasodilation in a variety of vascular beds including cerebral vasculature50,51,52,53,54. This work aligns with previous studies showing a robust dilatory effect of CGS-2168050. Ngai and colleagues (2001) found that CGS-21680 induced significant dose-dependent vasodilation of cerebral arterioles55. Further, using the selective A2A antagonist ZM-241385, they were able to inhibit the dilatory response to both CGS-21680 and adenosine. They concluded that the A2A receptor was responsible for the adenosine-induced dilatory effect they saw in rat cerebral arterioles.
Caffeine has been shown to display vasoactive properties through a number of molecular systems, including adenosine30. Adenosine’s actions are mediated by specific cell surface receptors coupled to G proteins classified as A1, A2A, A2B, and A356. Caffeine acts as a competitive antagonist of the A1 and A2A receptors. However, caffeine also influences cerebrovascular dynamics through mechanisms independent of adenosine receptors. Caffeine can liberate calcium from intracellular stores and modulate calcium channels and increase prostaglandin E2 (PGE2) synthesis57,58,59. Previous studies have found that seizure-induced vasoconstriction is mediated by vasoactive prostanoids, such as PGE212,16. They found that ibuprofen, a cyclooxygenase-2 blocker prevented postictal vasoconstriction, hypoperfusion and brain tissue hypoxia. They also showed that ibuprofen did not influence seizure duration, indicating that its effects were attributable to the neurovascular phenomenon and not a seizure-altering mechanism17. Caffeine’s ability to increase the production of PGE2 coupled with seizure induced PGE2 activation could potentially culminate to produce fatal outcomes when it occurs in the brainstem and may have occurred in our models.
Sudden unexpected death in epilepsy continues to be a major cause of death in people with drug resistant epilepsy60,61. Our data support a detrimental effect of caffeine on SUDEP pathophysiology in two models of seizure-induced death. In our hands acute administration of caffeine significantly decreased oxygen in both the hippocampus and brainstem and accelerated time to death. The ubiquitous nature of caffeine consumption makes this finding of considerable clinical significance. Caffeine consumption is an easily modifiable risk factor for SUDEP and reveals a promising strategy to reduce death. Additionally, the present study points to the utility of A2A agonists as a promising tool to reduce SUDEP risk.
Methods
Animals
Animal care and use procedures were carried out in accordance with standards set by the Canadian Council for Animal Care and ARRIVE guidelines. Experimental protocols were approved by the Health Sciences Animal Care Committee at the University of Calgary (AC20-0170). Male C57BL/6J mice aged 7 weeks (P50) and weighing 25–32 g were obtained from Charles River Laboratories (Wilmington, MA, USA). Male Kcna1−/− mice on a C3HeB/FeJ background were obtained from heterozygous breeding colonies established at the University of Calgary. Animals were housed in a temperature-controlled environment with ad libitum access to food and water. All procedures complied with ARRIVE guidelines.
Acute experiment surgeries
Brain implant surgery
Male C57BL/6J mice on postnatal day 50 were anesthetized with 5% isoflurane and maintained on 2% isoflurane mixed with 100% oxygen. The skull was secured in a stereotaxic apparatus (David Kopf Instruments) using ear and incisor bars. Diluted lidocaine (0.5% 1:4 lidocaine, saline) was injected subcutaneously over the incision site followed by a sagittal incision along the midline of the head. Burr holes were drilled in the skull at stereotaxic coordinates. Bipolar depth electrodes were implanted unilaterally into the dorsal hippocampus at these coordinates relative to bregma: (AP) − 2.2, (ML) − 2.5, (DV) − 2.0 and the pre-Bötzinger complex at: (AP) − 7.0, (ML) − 1.4, (DV) − 4.1. Oxygen sensing optodes (Oyxylite, Oxford Optronix) were implanted in the ipsilateral ventral hippocampus at: (AP) − 1.5, (ML) − 0.8, (DV) − 1.5 and in the contralateral pre-Bötzinger complex at: (AP) − 7.0, (ML) + 1.4, (DV) 4.1. A 22-gauge infusion guide cannula (Plastics One, Roanoke, VA) was implanted in the contralateral dorsal hippocampus at: (AP) − 2.7, (ML) + 3.0, (DV) − 3.0. Electrodes and probes were secured to the skull using Metabond Quick Adhesive (C&B) and dental acrylic. Mice were administered injectable buprenorphine (0.05 mg/kg) every 12 h for 3 days post-operatively and mice recovered for 3 days before diaphragmatic surgery.
Diaphragmatic surgery
On the same day after brain implantation surgery, mice were maintained on 2% isoflurane mixed with 100% oxygen. Diluted lidocaine (0.5% 1:4 lidocaine, saline) was injected subcutaneously at the incision site on the dorsal segment of the ribs just below the ribcage. Electrode wires were implanted as described by Pagliardini and colleagues into the diaphragm to record phrenic nerve activity62. In brief, multi-stranded Teflon-coated stainless-steel electrode wire (#AS633, Cooner Wire) were passed through the diaphragm using a suture needle and secured in place by a knot. A second electrode wire was placed 1 mm away from the first to provide a bipolar recording. The electrode ends were then tunneled under the skin to an incision on the animal’s dorsum, between the scapulae. The uninsulated ends of the electrode wires were then attached to male amphenol pins and secured in a 9-pin ABS socket (Ginder Scientific) that protruded from the back. The incision sites were sutured, and the mice recovered. Mice were administered injectable buprenorphine (0.05 mg/kg) every 12 h for 3 days post-operatively and were given 7 days to recover before beginning acute experiments.
Chronic experiment surgery
Intracerebroventricular surgery
Male Kcna1−/− mice on postnatal day 26, mice were anesthetized with 5% isoflurane and maintained on 2% isoflurane mixed with 100% oxygen. The skull was secured in a stereotaxic apparatus using ear and incisor bars. Diluted lidocaine (0.5% 1:4 lidocaine, saline) was injected subcutaneously over the incision site followed by a sagittal incision along the midline of the head. The incision was then extended over the dorsum of the mouse posterior to the scapulae. Haemostats were introduced subcutaneously to widen incision and produce dead space for pump insertion. A burr hole was made over the right lateral ventricle at these coordinates relative to bregma: (AP) + 0.3, (ML) − 1.0, (DV) − 3.030. The infusion cannula was secured to the skull using Metabond Quick Adhesive (C&B) and dental acrylic. Mice were administered injectable buprenorphine (0.05 mg/kg) every 12 h for 3 days post-operatively and mice were allowed to recover for 3 days before starting the experiment on P30. The patency and placement of the cannula was verified at the end of the experiments using methylene blue dye (Sigma-Aldrich) administration.
Drug administration
Acute drug administration
After a 7-day recovery period from surgery, male C57BL/6J mice were administered either caffeine (10 mg/kg), theophylline (10 mg/kg), N6-Cyclopentyladenosine (N6) (1 mg/kg) or CGS-21680 (1 mg/kg) or vehicle DMSO for lipophilic drugs or saline for hydrophilic drugs) intraperitoneal (i.p) 30 min prior to infusion of kainic acid. Kainic acid (1.4 μg in 0.4 μL) was infused unilaterally through an intrahippocampal cannula into the right dorsal hippocampus at 0.1 μL/min through a 1 μL Hamilton syringe (Hamilton Robotics, Reno NV) and micro-syringe pump (Harvard Apparatus, model 55-2222, Canada). All drugs were purchased from Cayman Chemicals. In acute experiments, local field potential, EMG and brain pO2 were recorded continuously and time to death following infusion was recorded.
Chronic caffeine in drinking water
Male Kcna1−/− mice on a C3HeB/FeJ congenic background were used. These mice carry a deletion of the Kcna1 gene on chromosome 6 and are lacking functional voltage-gated Kv1.1 channels. Starting the experiment on postnatal day 30, male mice received either water (vehicle) or water with 0.5 mg/mL caffeine (Sigma-Aldrich). Mice were monitored every 12 h and date of death recorded. Caffeine was protected from light and replaced every 12 h to ensure photostability and solubility.
Chronic intracerebroventricular CGS-21680 administration
Male Kcna1−/− mice were implanted with Alzet 42-day mini-pumps (model 2006; Durect, Cupertino) which continuously delivered CGS-21680 (or vehicle) into the left lateral ventricle (0.2 mg/mL, pumping rate 0.15 μL/h) this dose was selected based on pilot data suggesting toxicity and lethality at higher concentrations. CGS-21680 was dissolved in DMSO, PEG and 100% ETOH (50:40:10) (Cayman Chemicals). Mice were monitored every 12 h and date of death recorded.
Physiological recordings
Absolute oxygen recordings
Absolute oxygen recordings were measured using an implantable fibre-optic oxygen-sensing probe (Oxylite, Oxford Optronics). These probes emit a short burst of LED light to a platinum fluorophore at the tip of the probe. When oxygen molecules collide with the fluorophore the light emitted by the probe is quenched and is registered as a decay in luminescence which is relayed to the terminal for display. The probe allows for accurate and continuous readings of oxygen in brain parenchyma in the awake and freely moving animal. The optode can measure a volume of 500 µm3 between 0 and 90 mmHg. Unless otherwise stated, sample rate of 60 light pulses/min were used at 1 Hz. Severe hypoxia is defined as < 10 mmHg because oxygen levels at or below this threshold are associated with brain injury resulting from cellular damage and death leading to worse clinical outcomes.
Electromyography recordings and analysis
The EMG was analyzed using previously published methods14. Heart rate and respiratory rate was obtained from raw EMG signal as a post-processing procedure. To obtain respiratory rate, the signal was low pass filtered below 0.01 Hz using a fifth-order Butterworth filter to remove signal drift and to determine during which part of the recordings the mouse was alive. Next, the raw EMG data was again low pass filtered below 4 Hz to extract respiratory information. The duration of the recording while the mouse was alive was the analyzed for individual breaths, done by applying a 1D data peak detection algorithm (peakutils.peak). Local maxima larger than a normalized threshold height were identified as breaths (the exact amplitude being dependent on the signal strength in the individual dataset). Heart rate data was processed by using custom algorithm (Python 2.7.12, scipy 0.18.1, peakutils 1.1.0). The signal was low pass filtered at 5 Hz using a fifth-order Butterworth filter to extract respiration and movement artifact. This low frequency signal was subtracted from the raw EMG to remove low frequency components, leaving only high frequency components such as heartbeats and signal noise. Individual heartbeats were determined by identifying local maxima using a 1D data peak detection algorithm (peakutils.peak). Only peaks over a certain threshold amplitude defined relative to the maximum amplitude in the signal (the exact threshold being dependent on the overall signal strength in the individual dataset) were accepted as heartbeats. A minimum distance between peaks was defined as 12 ms for heartbeats, as heartbeats at a higher frequency would not be physiologically possible. Breathing and heart rate were obtained by calculating the time difference between peaks and creating a moving average across the entire data set.
Statistics
All statistics were calculated using GraphPad Prism version 9 (GraphPad Software, Inc., San Diego, CA, USA, 2003) Unpaired (comparisons between animals) t-tests were used for experiments with only two groups. When more than two groups were compared either a one-way ANOVA, or two-way ANOVA were applied. Survival curves were analyzed by Kaplan–Meier survival estimates using the log-rank test. A priori normality testing (D’ Agostino and Pearson) was followed by a student’s t-test. Significance thresholds were set to: *p < 0.05. Unless otherwise stated, values were expressed as means ± standard error of the mean (SEM).
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Devinsky, O. Sudden, unexpected death in epilepsy. N. Engl. J. Med. 365(19), 1801–1811 (2011).
Sowers, L. P., Massey, C. A., Gehlbach, B. K., Granner, M. A. & Richerson, G. B. Sudden unexpected death in epilepsy: Fatal post-ictal respiratory and arousal mechanisms. Respir. Physiol. Neurobiol. 189(2), 315–323 (2013).
Terra, V. C., Cysneiros, R., Cavalheiro, E. A. & Scorza, F. A. Sudden unexpected death in epilepsy: From the lab to the clinic setting. Epilepsy Behav. 26(3), 415–420 (2013).
Ficker, D. M. et al. Population-based study of the incidence of sudden unexplained death in epilepsy. Neurology 51(5), 1270–1274 (2007).
Nashef, L., Hindocha, N. & Makoff, A. Risk factors in sudden death in epilepsy (SUDEP): The quest for mechanisms. Epilepsia 48(5), 859–871 (2007).
Surges, R., Thijs, R. D., Tan, H. L. & Sander, J. W. Sudden unexpected death in epilepsy: Risk factors and potential pathomechanisms. Nat. Rev. Neurol. 5(9), 492–504 (2009).
Thurman, D. J., Hesdorffer, D. C. & French, J. A. Sudden unexpected death in epilepsy: Assessing the public health burden. Epilepsia 55(10), 1479–1485 (2014).
Stewart, M. et al. Obstructive apnea due to laryngospasm links ictal to postictal events in SUDEP cases and offers practical biomarkers for review of past cases and prevention of new ones. Epilepsia 58(6), e87-90 (2017).
Friedman, D. et al. Cardiac arrhythmia and neuroexcitability gene variants in resected brain tissue from patients with sudden unexpected death in epilepsy (SUDEP). NPJ Genom. Med. 3(1), 9 (2018).
Petrucci, A. N., Joyal, K. G., Purnell, B. S. & Buchanan, G. F. Serotonin and sudden unexpected death in epilepsy. Exp. Neurol. 325, 113145 (2020).
Ryvlin, P. et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): A retrospective study. Lancet Neurol. 12(10), 966–977 (2013).
Farrell, J. S. et al. Postictal behavioural impairments are due to a severe prolonged hypoperfusion/hypoxia event that is COX-2 dependent. Elife 5, e19352 (2016).
Gaxiola-Valdez, I. et al. Seizure onset zone localization using postictal hypoperfusion detected by arterial spin labelling MRI. Brain 140(11), 2895–2911 (2017).
Li, E. et al. CT perfusion measurement of postictal hypoperfusion: Localization of the seizure onset zone and patterns of spread. Neuroradiology 61, 991–1010 (2019).
Liu, J. et al. Postictal brainstem hypoperfusion and risk factors for sudden unexpected death in epilepsy. Neurology 95(12), e1694–705 (2020).
Farrell, J. S. et al. In vivo endocannabinoid dynamics at the timescale of physiological and pathological neural activity. Neuron 109(15), 2398-2403.e4 (2021).
George, A. G. et al, Sudden unexpected death in epilepsy is prevented by blocking postictal hypoxia. Neuropharmacology. https://doi.org/10.1016/j.neuropharm.2023.109513 (2023).
Phillips, T. J., Gom, R. C., Wolff, M. D. & Teskey, G. C. Caffeine exacerbates postictal hypoxia. Neuroscience 422, 32–43 (2019).
Nehlig, A. Are we dependent upon coffee and caffeine? A review on human and animal data. Neurosci. Biobehav. Rev. 23(4), 563–576 (1999).
van Koert, R. R., Bauer, P. R., Schuitema, I., Sander, J. W. & Visser, G. H. Caffeine and seizures: A systematic review and quantitative analysis. Epilepsy Behav. 80, 37–47 (2018).
Kaufman, K. & Sachdeo, R. Caffeinated beverages and decreased seizure control. Seizure 12, 519 (2003).
Trenité, D. G. et al. Consensus on diagnosis and management of JME: From founder’s observations to current trends. Epilepsy Behav. 28, S87-90 (2013).
Wassenaar, M., Kasteleijn-NolstTrenité, D. G., de Haan, G. J., Carpay, J. A. & Leijten, F. S. Seizure precipitants in a community-based epilepsy cohort. J. Neurol. 261(4), 717–724 (2014).
Akins, V. T., Weragalaarachchi, K., Picardo, M. C., Revill, A. L. & Del Negro, C. A. Morphology of Dbx1 respiratory neurons in the preBötzinger complex and reticular formation of neonatal mice. Sci. Data 4, 170097 (2017).
Oddo, M. et al. Brain hypoxia is associated with short-term outcome after severe traumatic brain injury independently of intracranial hypertension and low cerebral perfusion pressure. Neurosurgery 69(5), 1037–1045 (2011).
Rho, J. M., Tempel, B. L. & Schwartzkroin, P. A. Developmental seizure susceptibility of kv1. 1 potassium channel knockout mice. Dev. Neurosci. 21(3–5), 320–327 (1999).
Shenkin, H. A. Effects of various drugs upon cerebral circulation and metabolism in man. J. Appl. Physiol. 3, 447–465 (1951).
Scheinberg, P. & Jayne, H. W. Factors influencing cerebral blood flow and metabolism. Circulation 5, 225–226 (1952).
Mathew, R. J. & Wilson, W. H. Caffeine-induced changes in cerebral circulation. Stroke 16, 814–817 (1985).
Echeverri, D., Montes, F. R., Cabrera, M., Galán, A. & Prieto, A. Caffeine’s vascular mechanisms of action. Int. J. Vasc. Med. 2010, 1–10 (2010).
Shen, H. Y., Li, T. & Boison, D. A novel mouse model for sudden unexpected death in epilepsy (SUDEP): Role of impaired adenosine clearance. Epilepsia 51(3), 465–468 (2010).
Temple, J. L. et al. The safety of ingested caffeine: A comprehensive review. Front. Psych. 8, 80 (2017).
Bragin, A., Engel, J. Jr., Wilson, C. L., Vizentin, E. & Mathern, G. W. Electrophysiologic analysis of a chronic seizure model after unilateral hippocampal KA injection. Epilepsia 40(9), 1210–1221 (1999).
Arkhipov, V., Kulesskaja, N. & Lebedev, D. Behavioral perseveration and impairment of long-term memory in rats after intrahippocampal injection of kainic acid in subconvulsive dose. Pharmacol. Biochem. Behav. 88(3), 299–305 (2008).
Rusina, E., Bernard, C. & Williamson, A. The kainic acid models of temporal lobe epilepsy. Eneuro 8(2), 337 (2021).
Lee, K. et al. Assessment of brain oxygenation imbalance following soman exposure in rats. Neurotoxicology 65, 28–37 (2018).
Wolff, M. D., Farrell, J. S., Scantlebury, M. H. & Teskey, G. C. Dynamic oxygen changes during status epilepticus and subsequent endogenous kindling. Epilepsia 61(7), 1515–1527 (2020).
Makino, K., Tanaka, T. & Yonemasu, Y. Regional cerebral blood flow and kainic acid-induced focal limbic seizures in cats. Epilepsy Res. 2(4), 260–268 (1988).
Hayward, N. M., Ndode-Ekane, X. E., Kutchiashvili, N., Gröhn, O. & Pitkänen, A. Elevated cerebral blood flow and vascular density in the amygdala after status epilepticus in rats. Neurosci. Lett. 484(1), 39–42 (2010).
Choy, M. et al. Cerebral blood flow changes during pilocarpine-induced status epilepticus activity in the rat hippocampus. Exp. Neurol. 225(1), 196–201 (2010).
Smeland, O. B., Hadera, M. G., McDonald, T. S., Sonnewald, U. & Borges, K. Brain mitochondrial metabolic dysfunction and glutamate level reduction in the pilocarpine model of temporal lobe epilepsy in mice. J. Cereb. Blood Flow Metab. 33(7), 1090–1097 (2013).
Villa, B. R. et al. Postictal hypoxia involves reactive oxygen species and is ameliorated by chronic mitochondrial uncoupling. Neuropharmacology 33(7), 1090–1097 (2023).
Rho, J. M. & Boison, D. The metabolic basis of epilepsy. Nat. Rev. Neurol. 18(6), 333–347 (2022).
Zhang, P., Bannon, N. M., Ilin, V., Volgushev, M. & Chistiakova, M. Adenosine effects on inhibitory synaptic transmission and excitation–inhibition balance in the rat neocortex. J. Physiol. 593(4), 825–841 (2015).
Blood, A. B., Hunter, C. J. & Power, G. G. The role of adenosine in regulation of cerebral blood flow during hypoxia in the near-term fetal sheep. J. Physiol. 543(3), 1015–1023 (2002).
Boison, D. Adenosinergic signaling in epilepsy. Neuropharmacology 104, 131–139 (2016).
Liu, Y. J. et al. Research progress on adenosine in central nervous system diseases. CNS Neurosci. Ther. 25(9), 899–910 (2019).
Fukuda, M., Suzuki, Y., Hino, H. & Ishii, E. Over-activation of adenosine A2A receptors and sudden unexpected death in epilepsy. Epilepsy Behav. 23(3), 387–388 (2012).
Faingold, C. L. & Feng, H. J. A unified hypothesis of SUDEP: Seizure‐induced respiratory depression induced by adenosine may lead to SUDEP but can be prevented by autoresuscitation and other restorative respiratory response mechanisms mediated by the action of serotonin on the periaqueductal gray. Epilepsia 64(4), 779–796 (2023).
Nayeem, M. A. et al. Role of CYP epoxygenases in A2AAR-mediated relaxation using A2AAR-null and wild-type mice. Am. J. Physiol.-Heart Circ. Physiol. 295(5), H2068–H2078 (2008).
Nayeem, M. A. et al. High-salt diet enhances mouse aortic relaxation through adenosine A2A receptor via CYP epoxygenases. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 296(3), R567–R574 (2009).
Hein, T. W., Belardinelli, L. & Kuo, L. Adenosine A2A receptors mediate coronary microvascular dilation to adenosine: Role of nitric oxide and ATP-sensitive potassium channels. J. Pharmacol. Exp. Ther. 291(2), 655–664 (1999).
Carroll, M. A. et al. Adenosine2A receptor vasodilation of rat preglomerular microvessels is mediated by EETs that activate the cAMP/PKA pathway. Am. J. Physiol.-Renal Physiol. 291(1), F155–F161 (2006).
Khayat, M. T. & Nayeem, M. A. The role of adenosine A2A receptor, CYP450s, and PPARs in the regulation of vascular tone. BioMed. Res. Int. 2017, 1–13 (2017).
Ngai, A. C., Coyne, E. F., Meno, J. R., West, G. A. & Winn, H. R. Receptor subtypes mediating adenosine-induced dilation of cerebral arterioles. Am. J. Physiol.-Heart Circ. Physiol. 280(5), H2329–H2335 (2001).
Jacobson, K. A. & Gao, Z. G. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 5(3), 247–264 (2006).
Hughes, A. D., Hering, S. & Bolton, T. B. The action of caffeine on inward barium current through voltage-dependent calcium channels in single rabbit ear artery cells. Pflugers Arch. 416(4), 462–466 (1990).
Parker, I. & Ivorra, I. Caffeine inhibits inositol trisphosphate-mediated liberation of intracellular calcium in Xenopus oocytes. J. Physiol. 433(1), 229–240 (1991).
Umemura, T. et al. Effects of acute administration of caffeine on vascular function. Am. J. Cardiol. 98(11), 1538–1541 (2006).
Holst, A. G. et al. Epilepsy and risk of death and sudden unexpected death in the young: A nationwide study. Epilepsia 54(9), 1613–1620 (2013).
Massey, C. A., Sowers, L. P., Dlouhy, B. J. & Richerson, G. B. Mechanisms of sudden unexpected death in epilepsy: The pathway to prevention. Nat. Rev. Neurol. 10(5), 271–282 (2014).
Pagliardini, S., Greer, J. J., Funk, G. D. & Dickson, C. T. State-dependent modulation of breathing in urethane-anesthetized rats. J. Neurosci. 32(33), 11259–11270 (2012).
Acknowledgements
We thank Drs. Sylvia Pagliardini and Greg Funk for teaching us the heart and respiratory recording surgical procedures.
Funding
Canadian Institutes of Health Research Grant (PJT-152956) to G.C.T. and SUDEP Aware to A.G.G.
Author information
Authors and Affiliations
Contributions
A.G.G.: involved in study conceptualization, oversight of data collection; data analysis; data visualization; writing original draft. A.F.: involved study conceptualization; data collection; data analysis; writing-review and editing. S.A.H.: involved in data collection; writing-review and editing. R.C.G.: involved in data analysis; writing-review and editing. G.C.T.: involved in study conceptualization; oversight of data collection; writing-review and editing; funding acquisition; supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
George, A.G., Federico, A., Gom, R.C. et al. Caffeine exacerbates seizure-induced death via postictal hypoxia. Sci Rep 13, 14150 (2023). https://doi.org/10.1038/s41598-023-41409-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-41409-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.