Abstract
A synthetic heme possessing immobilized fluorine atoms as peripheral side chains has been incorporated into the apoprotein of sperm whale myoglobin (Mb), and the field dependence of the line width of 19F NMR signals observed for the protein with S values ranging from 0 to 5/2 was analyzed in order to gain a quantitative insight into the 19F transverse relaxation mechanism in Mb with various magnetic properties. In the cases of deoxy (S=2) and met-aquo (S=5/2) Mbs, the significant contribution of Curie spin relaxation to the 19F transverse relaxation was demonstrated and analysis of the Curie spin relaxation was useful to estimate the overall correlation time of the protein.
Similar content being viewed by others
Introduction
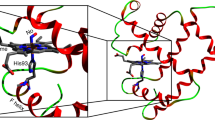
The analysis of nuclear relaxation has provided a wealth of information about structural and dynamic properties of molecules. In particular, in the case of metalloproteins carrying unpaired electron(s), the dynamic nature of a molecule is sharply manifested in paramagnetic relaxation observed on paramagnetically shifted nuclear magnetic resonance (NMR) signals.1, 2, 3, 4 In the present study, we have analyzed the field dependence of the line widths of NMR signals of sperm whale myoglobins (Mbs) with a variety of magnetic properties in order to elucidate field-dependent properties of the paramagnetic relaxation. Mb is an oxygen-binding hemoprotein with a molecular weight of ∼17 kDa (Figure 1a), and a single heme (Figure 1b) is embedded in its protein matrix. The side chain of a His residue is coordinated to the heme iron as an axial ligand, and an exogenous ligand, such as not only the dioxygen molecule (O2), but also a carbon monoxide (CO) or water molecule (H2O), and cyanide ion (CN−) is bound to the iron on the side of the heme opposite the axial His (Figure 1d). The heme iron in Mb is either in the ferrous or ferric state, and hence, depending upon the degree of spin pairing of electrons in the 3d orbitals, ferrous heme iron can have 4, 2 or 0 unpaired electrons, corresponding to total spin quantum number S=2, 1 or 0, respectively, and for ferric heme iron S=5/2, 3/2 or 1/2 with 5, 3 or 1 unpaired electron, respectively. The spin state of Mb depends on the chemical nature of the exogenous ligand. For ferrous heme iron, the deoxy (no ligand) form (deoxyMb) is penta-coordinated with a high-spin configuration, S=2, and the oxy (MbO2) or carbonmonoxy form (MbCO) possesses a low-spin configuration, S=0 (Figure 2). On the other hand, the coordination of H2O to ferric heme iron gives high-spin state S=5/2 (metMb), low-spin state S=1/2 being obtained with CN− (MbCN−) (Figure 2).
By virtue of the absence of interfering background signals, the introduction of fluorine atom(s) into the peripheral side chain(s) of the heme facilitates the observation of their signals in various oxidation, spin and ligation states of the protein.5, 6, 7, 8, 9, 10, 11 19F is a 100% abundant nucleus with nuclear spin I=1/2 and, because of a relatively high gyromagnetic ratio, 19F NMR is ∼83% as sensitive as 1H NMR. In addition, because of the wider spectral range for 19F NMR than 1H NMR, individual signals will be better resolved on 19F NMR.12, 13 A ring-fluorinated heme, 13,17-bis(2-carboxylatoethyl)-3,7-difluoro-2,8,12,18-tetramethyl-porphyrinatoiron(III) (3,7-DF (Figure 1c)),5 has been incorporated into Mb, and the field dependence of 19F NMR signals arising from MbCO (S=0), MbCN− (S=1/2), deoxyMb (S=2) and metMb (S=5/2) has been analyzed in order to evaluate the 19F relaxation mechanism in the proteins. Despite considerably large line widths of paramagnetically shifted signals, the high sensitivity and excellent signal resolution of 19F NMR allowed the observation of well-separated signals in the spectra of all the paramagnetic Mbs examined in the study. Furthermore, as the fluorine atoms introduced to the heme as peripheral side chains are dynamically fixed to the porphyrin ring, detailed analysis of the field dependence of the line widths of the 19F signals was useful to estimate the correlation time (τr) of the heme accommodated in the active site of the protein.
The nuclear relaxation rate (Robs) in a paramagnetic system is expressed as the sum of diamagnetic (Rdia) and paramagnetic (Rpara) terms,1

Rdia is written as the sum of the contributions of the dipole–dipole interaction  , chemical shift anisotropy
, chemical shift anisotropy  , and others
, and others  ,
,

The contributions of  to the nuclear spin–lattice (R1dia) and spin–spin (R2dia) relaxation rates (
to the nuclear spin–lattice (R1dia) and spin–spin (R2dia) relaxation rates ( and
and  , respectively) depend on the magnetic field strength and can be written as follows,14
, respectively) depend on the magnetic field strength and can be written as follows,14



where  is associated with the principal components of the chemical shift tensor, η is the asymmetric parameter of the molecule under consideration, and J(ωI) represents the spectral density function.
is associated with the principal components of the chemical shift tensor, η is the asymmetric parameter of the molecule under consideration, and J(ωI) represents the spectral density function.
On the other hand, Rpara is expressed as the sum of the contributions of metal-centered ( ) and ligand-centered (
) and ligand-centered ( ) dipolar terms, the contact hyperfine interaction (
) dipolar terms, the contact hyperfine interaction ( ), and Curie spin relaxation (
), and Curie spin relaxation ( ),1
),1

Using the Solomon–Bloembergen equations,15, 16 together with the expression of  ,17, 18 paramagnetic contributions to the nuclear spin–lattice and spin–spin relaxation rates (R1para and R2para, respectively) in a paramagnetic system are expressed by
,17, 18 paramagnetic contributions to the nuclear spin–lattice and spin–spin relaxation rates (R1para and R2para, respectively) in a paramagnetic system are expressed by






where, in the present case, rM and rL are the distance between the F and Fe atoms, and the F–C bond length, respectively, ρ is the unpaired electron density at the carbon and fluorine atoms at positions 3 and 7 on the porphyrin of 3,7-DF (Figure 1c),  is the apparent hyperfine coupling constant for 19F, ωI and ωS are the Larmor frequencies of 19F and electron, respectively, T1e and T2e are the electron longitudinal and transverse relaxation times, respectively, and τex is the electron exchange time. The other parameters are as usual. In large molecules with highly resolved NMR spectra, T1e,T2e<<τr, and at high magnetic field,
is the apparent hyperfine coupling constant for 19F, ωI and ωS are the Larmor frequencies of 19F and electron, respectively, T1e and T2e are the electron longitudinal and transverse relaxation times, respectively, and τex is the electron exchange time. The other parameters are as usual. In large molecules with highly resolved NMR spectra, T1e,T2e<<τr, and at high magnetic field,  , and at high magnetic field,
, and at high magnetic field,  , Equations (7) and (8) are reduced to the equations19, 20
, Equations (7) and (8) are reduced to the equations19, 20


Equation (14) dictates that R2para depends on the field strength owing to the contribution of  , the third term on the right-hand side of the equation. The nuclear spin–lattice (R1=R1dia+R1para) and spin–spin (R2 =R2dia+R2para) relaxation rates of a paramagnetic Mb are schematically plotted against the square of the applied field strength,
, the third term on the right-hand side of the equation. The nuclear spin–lattice (R1=R1dia+R1para) and spin–spin (R2 =R2dia+R2para) relaxation rates of a paramagnetic Mb are schematically plotted against the square of the applied field strength,  (Figure 3a), and a significant effect of
(Figure 3a), and a significant effect of  on R2 is demonstrated in the plots. In this study, analysis of the field dependence of the line widths of the signals allowed us to estimate
on R2 is demonstrated in the plots. In this study, analysis of the field dependence of the line widths of the signals allowed us to estimate  , which can be interpreted quantitatively in terms of the τr value.
, which can be interpreted quantitatively in terms of the τr value.
Plots of nuclear spin–lattice (R1) and spin–spin (R2) relaxation rates against the square of the magnetic field strength ( ) for a system with ωI2τr2>>1 (a) and ones of the R2dia,
) for a system with ωI2τr2>>1 (a) and ones of the R2dia, , and
, and  contributions to the line width (Δν1/2) against the magnetic field strength (Bo) for deoxyMb with S=2 (B). In (b), the R2dia and
contributions to the line width (Δν1/2) against the magnetic field strength (Bo) for deoxyMb with S=2 (B). In (b), the R2dia and  contributions were calculated from the results for MbCO with S=0, and R2para is the sum of R2dia,
contributions were calculated from the results for MbCO with S=0, and R2para is the sum of R2dia,  , and
, and  .
.
Experimental Procedures
Sample preparation
Mb was purchased as a lyophilized powder from Biozyme and used without further purification. The apoprotein of Mb was prepared at 4 °C according to the procedure of Teale.21 3,7-DF chloride was synthesized as previously described.5 Reconstitution of the apoprotein with heme was carried out by the standard procedure.5, 6 The reconstituted Mb was concentrated to ∼1 mM in an ultrafiltration cell (Amicon, Merck Millipore, Billerica, MA, USA) and the solvent was exchanged with 90% H2O/10%2H2O. A 10-fold molar excess of potassium cyanide was added to metMb to prepare MbCN−. MbCO was prepared by the injection of CO gas (Japan Air Gases) and the addition of Na2S2O4 (Wako Pure Chemical Industries, Ltd., Osaka, Japan). DeoxyMb was prepared from metMb, which had been evacuated and flushed with N2 gas (Japan air gases) several times, by the addition of Na2S2O4. The pH of the sample is the direct read of a pH meter (Horiba F-22 equipped with a Horiba type 6069-10c electrode). The p2H of the sample was adjusted using 0.2 M NaO2H or 2HCl.
19F NMR measurements
19F NMR spectra were recorded on either a Bruker AC-400 P or AVANCE-500 FT-NMR spectrometer operating at a 19F frequency of 376 (B0=9.4 T) or 471 (B0=11.7 T) MHz, respectively. A typical spectrum consisted of 20 k transients with a 100 kHz spectral width and 8 k data points. The signal-to-noise ratio of the spectrum was improved by apodization, which introduced 20–100 Hz line broadening. The NMR spectra were processed using XWIN-NMR version 3.5 (Bruker BioSpin, Karlsruhe, Germany) and line-shape analysis of the signals was performed using MestRe-c version 4.8.6.0 (Mestrelab Research) to obtain their line widths ( ). Chemical shifts are given in p.p.m. downfield from trifluoroacetic acid as an external reference.
). Chemical shifts are given in p.p.m. downfield from trifluoroacetic acid as an external reference.
Results and discussion
The 376 MHz 19F NMR spectra of various forms of Mb reconstituted with 3,7-DF are illustrated in Figure 4. The signal assignments were obtained on the basis of 19F–1H nuclear Overhauser effect connectivities and spectral comparison with Mbs reconstituted with other fluorinated hemes.5, 6 As described previously,5 the observation of two signals in the 19F NMR spectrum of MbCO (Figure 4) was the result of removal of the degeneracy of the magnetic environments of the two fluorine atoms of two-fold symmetric 3,7-DF through a heme–protein interaction. The shifts of the signals were closely related to the S value of the protein. In the spectrum of paramagnetic MbCN− (Figure 4), the 3- and 7-F signals were downfield-shifted by 54.7 and 21.2 p.p.m., respectively, relative to the corresponding ones of MbCO, at 25 °C, and the signals of deoxyMb and metMb were downfield-shifted to>200 and>300 p.p.m., respectively, relative to those of MbCO. On the other hand, the separation values of the two signals of MbCO, MbCN−, deoxyMb, and metMb at 25 °C were 2.4, 31.1, 18.5 and 33.9 p.p.m., respectively. The relatively large separation for the MbCN− signals is due to its large in-plane asymmetry in the heme electronic structure. Furthermore, the non-equivalence of the line widths between the 3- and 7-F signals of each Mb is predominantly due to the difference in the contribution of the contact interaction to spin–spin relaxation rate.20
The line widths of the signals ( ) ranged from ∼100 Hz for MbCO with S=0 to>4000 Hz for metMb with S=5/2. The considerable broadening of the signals with increasing S value demonstrated the significant contributions of the paramagnetic relaxation mechanisms to the R2 value (Figure 4). The line widths of the signals at 9.4 T were compared with those of the corresponding signals at 11.7 T (Table 1). For all the signals observed, the line widths at the higher field strength were larger than those at the lower field strength. In addition, the difference in the line width between the signals recorded at two different field strengths increased with increasing S value, indicating the significant contributions of the
) ranged from ∼100 Hz for MbCO with S=0 to>4000 Hz for metMb with S=5/2. The considerable broadening of the signals with increasing S value demonstrated the significant contributions of the paramagnetic relaxation mechanisms to the R2 value (Figure 4). The line widths of the signals at 9.4 T were compared with those of the corresponding signals at 11.7 T (Table 1). For all the signals observed, the line widths at the higher field strength were larger than those at the lower field strength. In addition, the difference in the line width between the signals recorded at two different field strengths increased with increasing S value, indicating the significant contributions of the  values to the R2 values of the signals.
values to the R2 values of the signals.
In the case of diamagnetic MbCO, the increase in the line width of the signals observed at higher magnetic field is attributed solely to the  contribution. As shown in Table 2, the differences in the
contribution. As shown in Table 2, the differences in the  values calculated from the
values calculated from the  values (
values ( ) yielded the
) yielded the  values. In order to calculate the
values. In order to calculate the  values,
values,  was assumed (see below), and hence equation (4) was reduced to
was assumed (see below), and hence equation (4) was reduced to  . The calculated
. The calculated  value for 3-F(or 7-F) is proportional to
value for 3-F(or 7-F) is proportional to  , where ωI1 and ωI2 are 376 and 471 MHz, respectively, and hence its
, where ωI1 and ωI2 are 376 and 471 MHz, respectively, and hence its  value at a given field strength could be calculated without the knowledge of the other parameters. The obtained values indicated that the
value at a given field strength could be calculated without the knowledge of the other parameters. The obtained values indicated that the  contributions to the
contributions to the  values of the signals at 9.4 T and 11.7 T are ∼35% and ∼50%, respectively.
values of the signals at 9.4 T and 11.7 T are ∼35% and ∼50%, respectively.
For paramagnetic MbCN− with S=1/2, the line width of the 3-F signal was larger by a factor of about two compared with that of the 7-F signal. The large difference in the  value between the signals of MbCN− is predominantly attributed to the
value between the signals of MbCN− is predominantly attributed to the  contribution, because the paramagnetic shift calculated using the shift of MbCO as a diamagnetic reference shift was much larger for the 3-F signal than the 7-F one, that is, 54.7 and 21.2 p.p.m. for the former and latter, respectively. The small
contribution, because the paramagnetic shift calculated using the shift of MbCO as a diamagnetic reference shift was much larger for the 3-F signal than the 7-F one, that is, 54.7 and 21.2 p.p.m. for the former and latter, respectively. The small  contributions to the R2obs values of the signals were clearly manifested in the relatively small field dependence of their
contributions to the R2obs values of the signals were clearly manifested in the relatively small field dependence of their  values.
values.
In the case of deoxyMb with S=2, the  values at 11.7 T increased by ∼200 Hz relative to those of the corresponding signals at 9.4 T. The large field dependence of the
values at 11.7 T increased by ∼200 Hz relative to those of the corresponding signals at 9.4 T. The large field dependence of the  values could be accounted for by the
values could be accounted for by the  and
and  contributions to the R2obs values, and the close correlation between the S value and the magnitude of the field dependence of the
contributions to the R2obs values, and the close correlation between the S value and the magnitude of the field dependence of the  value (Table 1) indicated that the latter contribution is much larger than the former. Assuming that the
value (Table 1) indicated that the latter contribution is much larger than the former. Assuming that the  contribution is independent of the oxidation, ligation and spin state of Mb, the
contribution is independent of the oxidation, ligation and spin state of Mb, the  value of deoxyMb could be estimated using the data obtained for MbCO, as summarized in Table 3. As shown in Table 3, the
value of deoxyMb could be estimated using the data obtained for MbCO, as summarized in Table 3. As shown in Table 3, the  value largely accounts for the R2para value, which predominantly determines the R2obs values of the signals of deoxyMb. Analysis of the
value largely accounts for the R2para value, which predominantly determines the R2obs values of the signals of deoxyMb. Analysis of the  contribution to the signals of deoxyMb, through the third term of equation (14), was useful for estimating the τr value of Mb. Values of ∼30 ns were obtained for the τr value, these values being larger compared with those reported previously, that is, ∼20 ns.22, 23, 24 The τr value estimated on 7-F signal exhibited larger deviation from the previously reported values than that on 3-F one, and hence the magnitude of the overestimation of the τr value using the present method could be related to the paramagnetic shifts of the signals. The observed paramagnetic shift is the sum of both contact and pseudo-contact shifts due to delocalization of unpaired electrons and magnetic anisotropy arising from unpaired electrons at heme Fe, respectively, and the separation of the two signals of a given paramagnetic Mb is attributed largely to the difference in the contact shift between them. The contact shift of the 3-F(or 7-F) signal is determined by the unpaired electron density in the pz (pπ) orbital of the pyrrole carbon atom to which the fluorine atom is attached, and hence the separation of the two signals is likely to reflect the non-equivalence in electronic nature between the two F–C bonds. Consequently, the larger separation of the two signals for deoxyMb than MbCO (Table 1) suggested that the non-equivalence in electronic environment between 3-F and 7-F in the former is larger than that in the latter. These results suggested a difference in electronic nature of 3(or 7)-F–C bond between the two Mb complexes, which in turn influences the
contribution to the signals of deoxyMb, through the third term of equation (14), was useful for estimating the τr value of Mb. Values of ∼30 ns were obtained for the τr value, these values being larger compared with those reported previously, that is, ∼20 ns.22, 23, 24 The τr value estimated on 7-F signal exhibited larger deviation from the previously reported values than that on 3-F one, and hence the magnitude of the overestimation of the τr value using the present method could be related to the paramagnetic shifts of the signals. The observed paramagnetic shift is the sum of both contact and pseudo-contact shifts due to delocalization of unpaired electrons and magnetic anisotropy arising from unpaired electrons at heme Fe, respectively, and the separation of the two signals of a given paramagnetic Mb is attributed largely to the difference in the contact shift between them. The contact shift of the 3-F(or 7-F) signal is determined by the unpaired electron density in the pz (pπ) orbital of the pyrrole carbon atom to which the fluorine atom is attached, and hence the separation of the two signals is likely to reflect the non-equivalence in electronic nature between the two F–C bonds. Consequently, the larger separation of the two signals for deoxyMb than MbCO (Table 1) suggested that the non-equivalence in electronic environment between 3-F and 7-F in the former is larger than that in the latter. These results suggested a difference in electronic nature of 3(or 7)-F–C bond between the two Mb complexes, which in turn influences the  value. Studies on reconstituted Mb possessing appropriate diamagnetic-model heme would be desirable to accurately estimate the
value. Studies on reconstituted Mb possessing appropriate diamagnetic-model heme would be desirable to accurately estimate the  value of deoxyMb. In addition, the precision in the determination of the τr value could be also improved by increasing the number of measurements at various field-strengths. On the basis of the estimated τr values, it could be at least concluded that 3,7-DF is dynamically fixed to the Mb protein, as has been demonstrated for hemes in various Mbs,25, 26 and hence the present system is in the slow-motion regime, which holds
value of deoxyMb. In addition, the precision in the determination of the τr value could be also improved by increasing the number of measurements at various field-strengths. On the basis of the estimated τr values, it could be at least concluded that 3,7-DF is dynamically fixed to the Mb protein, as has been demonstrated for hemes in various Mbs,25, 26 and hence the present system is in the slow-motion regime, which holds  . Finally, in the case of metMb, the field dependence of the line width of the signals was remarkably greater compared with that in the cases of the other Mbs. Extremely large broadening of the signals severely hampered accurate measurement of the
. Finally, in the case of metMb, the field dependence of the line width of the signals was remarkably greater compared with that in the cases of the other Mbs. Extremely large broadening of the signals severely hampered accurate measurement of the  values.
values.
With knowledge of the  and
and  values, the line width of the signals as a function of field strength could be estimated (Figure 3b). Owing to the factor S2(S+1)2 in Equation (14), the
values, the line width of the signals as a function of field strength could be estimated (Figure 3b). Owing to the factor S2(S+1)2 in Equation (14), the  value is expected to be larger by factors of 64 and 136 in deoxyMb (S=2) and metMb (S=5/2), respectively, relative to that in MbCN− (S=1/2), at a given field strength. On the basis of the
value is expected to be larger by factors of 64 and 136 in deoxyMb (S=2) and metMb (S=5/2), respectively, relative to that in MbCN− (S=1/2), at a given field strength. On the basis of the  value of 1490±660 s−1 obtained for deoxyMb at 9.4 T, the value for MbCN− was calculated to be ∼23 s−1 at the same field strength, which accounts for the line width of ∼7 Hz for the signals, confirming that the
value of 1490±660 s−1 obtained for deoxyMb at 9.4 T, the value for MbCN− was calculated to be ∼23 s−1 at the same field strength, which accounts for the line width of ∼7 Hz for the signals, confirming that the  contribution is negligibly small for the R2para value of the S=1/2 system, as has been described previously.6 Furthermore, abolition of the higher sensitivity of spectral measurements at higher magnetic field strength caused by the considerable broadening of the signals owing to the increased
contribution is negligibly small for the R2para value of the S=1/2 system, as has been described previously.6 Furthermore, abolition of the higher sensitivity of spectral measurements at higher magnetic field strength caused by the considerable broadening of the signals owing to the increased  contribution with increasing field strength was demonstrated for high-spin systems. Hence, spectral measurements of the proteins with large S values, such as deoxyMb and metMb, at higher magnetic field strength cannot make use of the benefits of high sensitivity and resolution, because of the considerable broadening of their signals owing to sharp increases in the
contribution with increasing field strength was demonstrated for high-spin systems. Hence, spectral measurements of the proteins with large S values, such as deoxyMb and metMb, at higher magnetic field strength cannot make use of the benefits of high sensitivity and resolution, because of the considerable broadening of their signals owing to sharp increases in the  and
and  contributions to spin–spin relaxation rate with increasing field strength (Figure 3b). Consequently, in order to record the spectra of paramagnetic molecules with better sensitivity and resolution, the magnetic field strength used for the measurements needs to be optimized according to their magnetic and dynamic properties.
contributions to spin–spin relaxation rate with increasing field strength (Figure 3b). Consequently, in order to record the spectra of paramagnetic molecules with better sensitivity and resolution, the magnetic field strength used for the measurements needs to be optimized according to their magnetic and dynamic properties.
Conclusion
A study on the field dependence of the line width of the NMR signals arising from immobilized fluorine atom(s) introduced to the active site of Mb with various S values has revealed the significant contributions of chemical shift anisotropy and Curie spin to 19F transverse relaxation. Curie spin relaxation dominates paramagnetic 19F transverse relaxation, and the overall correlation time of a protein could be estimated from the results of analysis of Curie spin relaxation.
References
Swift, T. J in NMR of Paramagnetic Molecules (eds) La Mar G. N., Horrocks W. D. Jr., Holm R. H.) Ch. 2 53–83 Academic Press, New York, (1973).
Yamamoto, Y. NMR Study of Paramagnetic Haemoproteins. Annu. Rep. NMR Spectrosc. 36, 1–77 (1998).
Bertini, I., Luchinat, C. & Parigi, G. Solution NMR of Paramagnetic Molecules, Elsevier, Amsterdam,, (2001).
Bertini, I. & Luchinat, C. NMR of Paramagnetic Molecules in Biological Systems, The Benjamin/Cummings Publishing Company, Inc., Menlo Park, (1986).
Yamamoto, Y., Hirai, Y. & Suzuki, A. 19F NMR study of protein-induced rhombic perturbations on the electronic structure of the active site of myoglobin. J. Biol. Inorg. Chem. 5, 455–462 (2000).
Hirai, Y., Yamamoto, Y. & Suzuki, A. 19F NMR study of the heme orientation and electronic structure in a myoglobin reconstituted with a ring-fluorinated heme. Bull. Chem. Soc. Jpn. 73, 2309–2316 (2000).
Poliart, C., Briand, J.-F., Tortevoie, F., Leroy, J., Simonneaux, G. & Bondon, A. High sensitivity of the fluorine NMR signals of difluorovinyl analogs of natural hemin: reconstituted heme proteins and self-exchange electron transfer in model compounds. Magn. Reson. Chem. 39, 615–620 (2001).
Yamamoto, Y., Nagao, S., Hirai, Y., Inose, T., Terui, N., Mita, H. & Suzuki, A. NMR Investigation of the Heme Electronic Structure in Deoxymyoglobin Possessing a Fluorinated heme. J. Biol. Inorg. Chem. 9, 152–160 (2004).
Hirai, Y., Nagao, S., Mita, H., Suzuki, A. & Yamamoto, Y. 19F NMR study on the heme electronic structure in oxy and carbonmonoxy reconstituted myoglobins. Bull. Chem. Soc. Jpn. 77, 1485–1486 (2004).
Nagao, S., Hirai, Y., Suzuki, A. & Yamamoto, Y. 19F NMR characterization of the thermodynamics and dynamics of the acid-alkaline transition in a reconstituted sperm whale metmyoglobin. J. Am. Chem. Soc. 127, 4146–4147 (2005).
Yamamoto, Y., Nagao, S. & Suzuki, A. 19F NMR Study of b-type haemoproteins. Modern Magn. Reson. 1, 531–538 (2006).
Sykes, B. & Hull, W. E. Fluorine nuclear magnetic resonance studies of proteins. Methods Enzymol. 49, 270–295 (1978).
Gerig, J. T. Fluorine NMR of proteins. Prog. NMR Spectrosc. 26, 293–370 (1994).
Abragam, A. Principles of Nuclear Magnetism, Oxford University Press, Oxford, (1961).
Solomon, I. Relaxation Processes in a System of Two Spins. Phys. Rev. 99, 559–565 (1955).
Bloembergen, N. Proton relaxation times in paramagnetic solutions. J. Chem. Phys. 27, 572–573 (1957).
Gueron, M. Nuclear relaxation in macromolecules by paramagnetic ions: a novel mechanism. J. Magn. Reson. 19, 58–66 (1975).
Vega, A. J. & Fiat, D. Nuclear relaxation processes of paramagnetic complexes. the slow motion case. Mol. Phys. 31, 347–355 (1976).
Unger, S. W., Jue, T. & La Mar, G. N. Proton NMR dipolar relaxation by delocalized spin density in low-spin ferric porphyrin complexes. J. Magn. Reson. 61, 448–456 (1985).
Yamamoto, Y. Analysis of 13C relaxation of heme peripheral methyl group in ferric low-spin myoglobin. J. Magn. Reson. B103, 72–76 (1994).
Teale, F. W. J. Cleanage of the Haem-protein Link by Acid Methylethylketone. Biochim. Biophys. Acta 35, 543 (1959).
Oldfield, E., Norton, R. S. & Alerhand, A. Studies of individual carbon sites of proteins in solution by natural abundance carbon 13 nuclear magnetic resonance spectroscopy: relaxation behavior. J. Biol. Chem. 250, 6368–6380 (1975).
Visscher, R. B. & Gurd, F. R. N. Rotational motions in myoglobin assesses by carbon 13 relaxation measurements at two magnetic field strengths. J. Biol. Chem. 250, 2238–2242 (1975).
Marshall, A. G., Lee, K. M. & Martin, P. W. Determination of rotational correlation time from perturbed angular correlations of γ rays: apomyoglobin reconstituted with 111Indium(III) mesoprotoporphyrin IX. J. Am. Chem. Soc. 102, 1460–1462 (1980).
Johnson, R. D., La Mar, G. N., Smith, K. M., Parish, D. W. & Langry, K. C. Solution deuterium NMR quadrupolar relaxation of heme mobility in myoglobin. J. Am. Chem. Soc. 111, 481–485 (1989).
Yamamoto, Y., Inoue, Y., Chujo, R. & Suzuki, T. 1H –NMR study of heme propionate mobility in the active site of myoglobin from Galeorhinus japonicus. Eur. J. Biochem. 189, 567–573 (1990).
Acknowledgements
We are indebted to Mr Taito Miyazaki for the assistance with recording the 19F NMR spectra. This work was supported by Grants-in-Aid for Scientific Research on Innovative Areas (No. 23108703, ‘π-Space’, and 23655151) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, the Yazaki Memorial Foundation for Science and Technology, and the NOVARTIS Foundation (Japan) for the Promotion of Science. The NMR spectra were recorded on a Bruker AVANCE-500 spectrometer at the Chemical Analysis Center, University of Tsukuba.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamamoto, Y., Nagao, S., Hirai, Y. et al. Field-dependent 19F NMR study of sperm whale myoglobin reconstituted with a ring-fluorinated heme. Polym J 44, 907–912 (2012). https://doi.org/10.1038/pj.2012.60
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2012.60
Keywords
This article is cited by
-
The secondary coordination sphere and axial ligand effects on oxygen reduction reaction by iron porphyrins: a DFT computational study
JBIC Journal of Biological Inorganic Chemistry (2016)