Abstract
The preparation and properties of crosslinking guar gum (GG) were studied by using ethanol as a solvent, epichlorohydrin as a crosslinking agent and sodium hydroxide as a catalyst. Some factors affecting the crosslinking degree of the crosslinked GG were investigated: reaction time, reaction temperature, amount of crosslinking agent, pH and solvent concentration. The sedimentation volume method was selected to determine the crosslinking degree of the crosslinked GG. The crosslinking degree was obviously influenced by the pH, reaction temperature and the crosslinking agent. The crosslinking improved alkali and acid resistance, retrogradation and viscosity stability of GG. The crosslinked GG has a significantly narrower melting transition as compared with GG.
Similar content being viewed by others
Introduction
Guar gum (GG) is extracted from the seed of the leguminous shrub Cyamopsis tetragonoloba. It is a naturally occurring galactomannan polysaccharide consisting of a linear chain of β-D-mannopyranose joined by β-(1–4) linkage with α-D-galactopyranosyl units attached by 1,6-links in the ratio of 1:2. It hydrates fairly rapidly in cold water, which gives highly viscous pseudoplastic solutions of generally greater low-shear viscosity. GG and its derivatives served as thickener, stabilizer, blister and flocculant are employed in the food, textile, pharmaceutical and paper industries due to their special properties.1, 2, 3, 4
Much effort has been devoted to the study of appropriate systems with special properties in order to avoid or minimize the side effects and to improve the efficacy of the therapy in the field of drug delivery in the last decades. Polysaccharides appear to be very attractive for their peculiar physico-chemical characteristics.5 GG is hydrophilic and swells in cold water, forming viscous colloidal dispersions or sols. This gelling property retards the release of the drug from the dosage form, increasing the likelihood of degradation in the colon. GG was found to be a colon-specific drug carrier in the form of matrix and compression-coated tablets as well as microspheres. The drug is inevitably dissolved, at least partially, because of the high pH level in the stomach. The modification of GG with borax or glutaraldehyde results in a product that is degraded by the enzymatic mixture of galactomannanase and α-galactosidase, possessing a higher buffer-uptake capacity as compared with native GG. The polysaccharides are crosslinked to a sufficient extent that they remain insoluble in water.6, 7, 8, 9, 10
GG can be modified by etherification, esterification, oxidation or graft. The derivatives of GG may compensate for the disadvantages of natural GG. For example, a carboxymethyl derivative may increase the hydrophilicity and solution clarity of the galactomannans and make it more soluble in an aqueous solution. The oxidized GG can be quickly hydrated becoming sticky or may even enter the solution. It was therefore difficult, if not impossible, to conduct oxidization reaction on GG without taking the materials into solution. A periodate oxidized gum can be maintained in a granular condition by carrying out the reaction in certain aqueous solvents or with very limited amounts of water. GG can be crosslinked with glutaraldehyde to reduce the tendency for swelling, which will allow it to maintain its degradable properties in the presence of typical colonic enzymes. It should be recognized that although glutaraldehyde is toxic, its toxicity could be reduced significantly after its crosslinking.11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21
In this investigation, we set out to examine preparation and property of crosslinking GG in order to improve the poor performance of the GG.
Materials and methods
Materials and instruments
GG, sodium hydroxide, epichlorohydrin, ethanol, potassium bromide and hydrochloric acid were purchased from china. All of these reagents were analytical.
WFJ 7200 visible-infrared spectrometer (UNICO (Shanghai) Instruments Co., Ltd., Shanghai, China); SHZ-C vacuum pump with circulated water system (Gongyi City Yuhua Instrument Co., Ltd., Gongyi, Henan Province, China); TDL80-2B desk centrifuge (ShangHai Anting Scientific Instrument Factory, Shanghai; China); fluidity meter (Shanghai Hongfu Instrument and apparatus Co., Ltd, Shanghai, China); 1010-2 electrothermal constant-temperature dry box (Jintan City Dadi Automation Instrument Factory, Jintan, Jiangsu Province, China); BL-320H electronic balance (Mettler Toledo instrument (Shanghai) Company Limited, Shanghai, China); NDJ-1A rotational viscometer (Shanghai Changji Geological Instrument Company Limited, Shanghai, China); IRPrestige-21 infrared spectrometer (Shimadzu Corporation, Kyoto, Japan); TGA Q50 V20.10 Build 36 thermogravimeric analyzer (TA Instruments, New Castle, DE, USA); DSC Q20 V24.4 Build 116 differential scanning calorimeter (TA Instruments, New Castle, DE, USA) are the instruments used.
Preparation of crosslinked GG
First a quantity of dry basis GG powder was weighed, and then alcohol and water were added to produce the GG slurry with a concentration of 35% (w/w) by mass basis. Finally, the slurry was put into the flask that was agitated continuously. The resulting slurry was heated to a certain temperature. After about 5 min, saturated oxyhydrogen sodium ethanol water solution was added to adjust the pH value of slurry to alkaline. And then a quantity of crosslinking agent was dropped into the GG slurry. At the same time the pH value of the slurry was kept constant by a saturated oxyhydrogen sodium ethanol water solution. When the reaction finished, the slurry was neutralized by 0.5 mol l−1 hydrochloric acid solution to a pH of 6.0–7.0. The slurry was vacuum filtered, then the filtered cake was washed by ethanol. The resultant cake was dried at 100 °C for about 4 h so that the moisture content could be less than 12%. The crosslinked GG was grounded and screened. The crosslinking GG was obtained in the end through all of these steps. The formula of GG reaction with epichlorohydrin in alkaline catalytic condition is as follows.

Sedimentation volume
One gram of a dry-based sample was weighed and put into a 100 ml beaker, and then 50 ml of distilled water was added. The pH of the GG slurry or crosslinking GG slurry was adjusted to a pH of 7.0 using 5% (w/w) sodium hydroxide or 5% (w/w) hydrochloric acid. The slurry was heated in a boiling water bath for 15 min. Distilled water was then added to bring the total weight to 50 g. The mixture was then stirred thoroughly and cooled to room temperature. The 10 ml of mixture was transferred to two 10 ml graduated cylinders, which were then sealed with a cap and centrifuged at 4000 r.p.m. for 15 min. The clear liquid was decanted, and the volume of the clear liquid was determined. The smaller the sedimentation volume was, the bigger the crosslinking degree would be. The sedimentation volume of GG was determined to be 10 ml, the point at which the gum completely dissolves in water. The sedimentation volume was expressed by the calculation as22

where V is volume of the clear liquid (ml).
Solubility and swelling power
A precisely weighed sample of 0.2 g was added to a preweighed 25-ml centrifugal tube, and then distilled water was added to give a total volume of water equivalent to 18 g. After that, the centrifugal tube was immediately placed in a temperature-controlled water bath at 85 °C and shaken continuously for 30 min. The centrifugal tube was then dried and placed on a balance followed by the addition of distilled water to bring to a total weight of 20 g. After capping, the centrifugal tube was centrifuged for 15 min at 3000 r.p.m. To measure solubility, the supernatant was transferred into an evaporating petri dish and dried overnight in a hot air oven at 105 °C. The dried residue was then cooled in desiccators and weighed for a soluble sample. To measure the swelling power, the residual supernatant was carefully removed and discarded. The bottle with sediment paste was then weighed to give the weight of swollen sample granules. The result was expressed by the calculation as22, 23


Freeze-thaw stability
Gum paste was prepared by mixing 4 g GG or crosslinking the GG (dry weight) and 100 g of distilled water and then gelatinized in a centrifuge tube by heating up to 95 °C and kept at this temperature for 15 min before cooling to ambient temperature. A 10 g sample of the gum paste that was precisely weighed was added into each of the preweighed 10ml centrifuge tubes, and these gum paste samples were frozen at 4 °C in a freezer for 24 h, then at −18 °C in a freezer for 24 h. All tubes were removed from the freezer and thawed at 30 °C in a water bath for 2 h. Three tubes from each thawing cycle of these samples were centrifuged at 3500 r.p.m. for 15 min. The clear liquid was decanted, and the residue was weighed. The separated water percentage was then calculated as the ratio of the weight of the liquid decanted to the total weight of the paste before centrifugation and multiplied by 100. The low separated water percentage means high freeze-thaw stability.24, 25, 26
Retrogradation
GG slurry, with a concentration of 1% (w/w) by dry crosslinking GG or GG, was produced by distilled water. A 50 ml slurry was put into a 100 ml beaker and gelatinized in a boiling bath for 10 min, and the volume of the slurry would be kept at this level during gelatinization. Gum paste was cooled to 25 °C. Gum paste transparency of supernatant was measured respectively at different standing times. The more transparent the gum paste was, the stronger the retrogradation would be, and vice versa.27, 28, 29, 30, 31
Acid and alkali resistance
Crosslinking GG solution with a concentration of 1% (w/w) by dry crosslinking GG was produced by distilled water. The crosslinking GG solution was heated in boiling water under constant agitation until it became a complete paste. Then the paste was put into cold water and cooled to room temperature. First, the paste fluidity was measured by fluid gauge under constant temperature, because fluidity is needed to discharge 40 ml of paste liquid over time. Second, the pH value of paste was adjusted to 10 or 3. The paste fluidity was measured again by fluid gauge under constant temperature. Fluidity changed slightly, and acid and alkali resistance were strong. The measurement method of acid and alkali resistance of GG was similar to that of crosslinking GG.32, 33, 34, 35
Stability of hot and cold viscosity
The hot and cold viscosities refer to the viscosity of a sample at 50 °C and 95 °C, respectively. The stability of viscosity is determined from

The fluctuation ratio of viscosity was expressed as

where max∣η−η′∣ is the maximum viscosity difference measured by keeping temperature constant for 60, 90, 120, 150 and 180 min, respectively, at 50 °C or 95 °C. η″ is viscosity measured by keeping temperature constant for 1 h at 95 °C or 50 °C.36, 37, 38
Infrared spectroscopy
IRPrestige-21 infrared spectrometer was used to record the infrared (IR) spectra within the range of 4000–400 cm−1. The IR spectra were recorded in solid state using a KB pellet method.
Thermal analysis
The thermal analysis of GG and crosslinked GG was carried out with a TGA Q50 V20.10 Build 36 thermogravimeric analyser and a DSC Q20 V24.4 Build 116 differential scanning calorimeter in nitrogen atmosphere. A heating rate of 10 °C min−1 was employed. To properly characterize the thermal properties of GG and crosslinked GG, the mixture need to be analyzed in a sealed sample pan in order to prevent the loss of water from the formulation during heating.
Statistical analysis
The data were expressed as means of triplicate determinations. Statistical significance was assessed with one-way analysis of variance using ORIGIN 7.5 for Windows program (OriginLab Software, Inc. Northampton, MA, USA). Treatment means were considered significantly different at P⩽0.05.
Results and Discussion
The effect of reaction temperature on sedimentation volume
The effect of reaction temperature on sedimentation volume of crosslinked GG is shown in Figure 1. Reaction conditions were as follows: GG 40 g and its water content 11.9%, pH 10, GG slurry concentration 35% (by dry GG, w/w), the required amount of epichlorohydrin 0.5% (by dry GG, w/w), reaction time 2.5 h, solvent (ethanol) concentration 85% (on basis of initial GG slurry, the following just the same).
From Figure 1, the sedimentation volume of crosslinked GG was markedly influenced by reaction temperature when reaction temperature was less than 65 °C. But the sedimentation volume changed little when reaction temperature was more than 65 °C. So the better reaction temperature is 70 °C.
The effect of reaction time on sedimentation volume
The effect of reaction time on sedimentation volume of crosslinked GG is shown in Figure 2. Reaction conditions were as follows: GG 40 g and its water content 11.9%, pH 10, GG slurry concentration 35%, the amount of epichlorohydrin 0.5%, reaction temperature 70 °C, solvent (ethanol) concentration 85%.
From Figure 2, it was evident that the sedimentation volume of crosslinked GG was markedly influenced by reaction time. The sedimentation volume of the crosslinked GG decreased when reaction time increased, if reaction time was less than 2.5 h. That was the increase of the crosslinking degree of crosslinked GG. The crosslinking degree was changed little when reaction time was more than 2.5 h. So the better reaction time is 2.5 h.
The effect of reaction pH on sedimentation volume
The effect of the reaction pH on sedimentation volume of crosslinked GG is shown in Figure 3. Reaction conditions were as follows: GG 40 g and its water content 11.9%, GG slurry concentration 35%, amount of epichlorohydrin 0.5%, reaction temperature 70 °C, reaction time 2.5 h, solvent (ethanol) concentration 85%.
From Figure 3, the crosslinking degree of the crosslinked GG increased when pH value increased, if the pH value was less than 10. But the crosslinking degree of product reduces when pH value is more than 10. So the better pH value is 10.
The effect of the amount of epichlorohydrin on sedimentation volume
The effect of the amount of epichlorohydrin (crosslinking agent) on sedimentation volume of crosslinked GG is shown in Figure 4. Reaction conditions were as follows: GG 40 g and its water content 11.9%, GG milk concentration 35%, pH 10, reaction temperature 70 °C, reaction time 2.5 h solvent (ethanol) concentration 85%.
From Figure 4, the crosslinking degree of the crosslinked GG increased when the amount of epichlorohydrin increased. The crosslinking degree of the crosslinked GG increased rapidly when the amount of epichlorohydrin was less than 0.5%, but it increased slowly when the amount of epichlorohydrin was more than 0.5%.
The effect of solvent concentration on sedimentation volume
The effect of solvent (ethanol) concentration on sedimentation volume of the crosslinked GG was shown in Figure 5. Reaction conditions were as follows: GG 40 g and its water content 11.9%, GG slurry concentration 35%, pH 10, amount of epichlorohydrin 0.5%, reaction temperature 70 °C, reaction time 2.5 h.
From Figure 5, the crosslinking degree of the crosslinked GG increased when ethanol concentration increased, if ethanol concentration was low. The crosslinking degree of the product reached the maximum when ethanol concentration value was 85%. But the crosslinking degree of the product decreased rapidly when ethanol concentration value was more than 85%. The phenomenon was explained by the following: when ethanol concentration was low, it meant that the GG slurry contained large amounts of water. As a result, the slurry viscosity increased because of GG particles absorbing water and swelling, further influencing the reaction of the GG with the crosslinking agent so that the crosslinking degree of the product decreased. However, when ethanol concentration was high, it meant that there was less water in the GG slurry, which would dissolve very little sodium hydroxide. The reaction could occur under the alkaline condition, so that the crosslinking agent reacted poorly with GG particles due to water reduction in the slurry. It resulted in a decrease of crosslinking degree in the product. So the better solvent concentration is 85%.
Freeze-thaw stability, alkali and acid resistance
Freeze-thaw stability, alkali and acid resistance of GG and crosslinked GG are shown in Table 1. From Table 1, the fluidity variation of the crosslinked GG was less than that of GG when pH of paste liquid was at 10 or 3. Its alkali resistance and acid resistance became stronger after the GG was crosslinked, and alkali and acid resistance increased when crosslinking degree was increased. But its freeze-thaw stability diminished.
Retrogradation
The retrogradation of GG and the crosslinked GG is shown in Figure 6. From Figure 6, its retrogradation improved, evidently, after the GG was crosslinked. The higher the degree of crosslinking, the stronger the retrogradation.
Solubility, swelling power and viscosity stability
Solubility, swelling power, viscosity stability of GG and the crosslinked GG are shown in Table 2. The viscosity stability included hot and cold viscosity stability. From Table 2, the fluctuation ratio of hot viscosity and cold viscosity, swelling power was reduced greatly after the GG was crosslinked, and increased when the crosslinking degree increased. Namely, its viscosity stability became better, but the swelling power and solubility decreased. The swelling property of GG was reduced by crosslinking it with increasing amounts of epichlorohydrin. The crosslinked GG with epichlorohydrin can potentially be used for the specific delivery of drugs with poor water solubility for the colon.
Infrared spectroscopy
Fourier transform IR of GG and crosslinked GG is shown in Figure 7. From Figure 7, there was basically no difference in shape between Fourier transform IR of GG and Fourier transform IR of the crosslinked GG. But the peak intensity was changed.
Thermal analysis
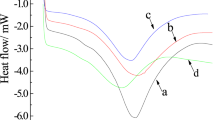
The thermogravimetric analysis (TGA) of the crosslinked GG is shown in Figure 8. The weight loss of GG and the crosslinked GG occurred in two distinct zones. The initial weight loss was due to the loss of the small amount of moisture in the sample and the second loss was due to the decomposition of polymer. The decomposition temperature of the crosslinked GG was 277.7 °C, GG 294.2 °C when the weight loss reached 25% on mass basis. The point temperature, where the GG TGA curve crossed the crosslinked GG TGA curve, was about 318 °C. When heating temperature was more than the intersect point temperature, the decomposition of GG occurred quicker than that of the crosslinked GG. It was obvious that the crosslinked GG was more stable compared with GG. The differential scanning calorimeter (DSC) differences between GG and crosslinked GG were more apparent in an overlay plot as displayed in Figure 9. The DSC profiles of GG and the crosslinked GG showed a single sharp endothermic peak between 40 and 170 °C at 126.3 °C for the crosslinked GG and 115.5 °C for the GG. It may be seen that crosslinked GG had a significantly narrower melting transition compared with GG. The thermal stability of GG was affected seriously by the crosslinking group.
Conclusions
The method of preparing the crosslinked GG was practical by using epichlorohydrin as the crosslinking agent, sodium hydroxide as the catalyst and ethanol as the solvent. The better reaction conditions for preparing the crosslinked GG are a reaction temperature of 70 °C, a reaction time of 2.5 h, pH 10 and an ethanol concentration of 85%. The crosslinking GG improved its alkali resistance, acid resistance, retrogradation and viscosity stability, but its freeze-thaw stability, swelling power and solubility decreased. The crosslinked GG has a significantly narrower melting transition as compared with GG. The thermal stability of GG was affected seriously by the crosslinking group.
References
Barre, L. L., Vaughan, A. S. & Sutton, S. J. On the solid-state ageing behavior of the polysaccharide, guar gum. J. Mater. Sci. 42, 5497–5507 (2007).
Nayak, B. R., Biswal, D. R., Karmakar, N. C. & Singh, R. P. Grafted hydroxypropyl guargum: development, characterization and application as flocculating agent. Bull. Mater. Sci. 25, 537–540 (2002).
Sharma, B. R., Dhuldhoya, N. C. & Merchant, U. C. Flocculants-an ecofriendly approach. J. Polym. Environ. 14, 195–202 (2006).
Emine, A. E. & Esra, I. Effects of pectin and guar gum on creaming stability, microstructure and rheology of egg yolk plasma-stabilized emulsions. Eur. Food Res. Technol. 231, 297–302 (2010).
Hoffman, A. S. Hydrogels for biomedical applications. Adv. Drug. Deliv. Rev. 54, 3–12 (2002).
Rubinstein, A. & Gliko-Kabir, I. Synthesis and swelling dependent enzymatic degradation of borax modified guar gum for colonic delivery purposes. S.T.P. Pharm. Sci. 5, 41–46 (1995).
Al-Saidan, S. M., Krishnaiah, Y. S. R., Satyanarayana, V., Bhaskar, P. & Karthikeyan, R. S. Pharmacokinetic evaluation of guar gum-based three-layer matrix tablets for oral controlled delivery of highly soluble metoprolol tartarate as a model drug. Eur. J. Pharm. Biopharm. 58, 697–703 (2004).
Sinha, V. R., Mittal, B. R. & Kumria, R. In vivo evaluation of time and site of disintegration of polysaccharide tablet prepared for colon-specific drug delivery. Int. J. Pharm 289, 79–85 (2005).
Gliko-Kabir, I., Yagen, B., Penhasi, A. & Rubinstein, A. Low swelling, crosslinked guar and its potential use as colon-specific drug carrier. Pharm. Res. 15, 1019–1025 (1998).
Chaurasia, M., Chourasia, M. K., Jain, N. K., Jain, A., Soni, V., Gupta, Y. & Jain, S. K. Cross-linked guar gum microspheres: a viable approach for improved delivery of anticancer drugs for the treatment of colorectal cancer. AAPS Pharm. Sci. Tech. 7, E1–E9 (2006).
Paranjothy, K. L. K. & Thampi, P. P. Synthesis of sodium carboxymethyl guar and its characterization. Indian Drugs 29, 404–407 (1992).
D’Melo, D. J. & Shenoy, M. A. Evaluation of mechanical properties of acrylated guar gum-unsaturated polyester composites. Polym. Bull. 61, 235–246 (2008).
Taunk, K . & Behari, K . Studies on graft copolymerization of 4-vinylpyridine onto guar gum. J. Appl. Polym. Sci. 84, 2380–2385 (2002).
Chauhan, K., Chauhan, G. S. & Ahn, J. H. Synthesis and characterization of novel guar gum hydrogels and their use as Cu2+ sorbents. Bioresour. Technol. 100, 3599–3603 (2009).
Prabhanjan, H., Gharia, M. M. & Srivastava, H. C. Synthesis of hydroxyethyl, hydroxypropyl, and carboxymethyl derivatives of Guar. Carbohydr. Polym. 11, 279–292 (1989).
Sharma, R. Guar gum grafting and its application in textile. Asian J. Exp. Sci. 19, 77–81 (2005).
Wenbo, W., Yuru, K. & Aiqin, W. Synthesis characterization and swelling properties of guar gum-g-polysodium acrylate-co-styrene muscovite super absorbent composites. Sci. Technol. Adv. Mater. 11, 1–10 (2010).
Mazhar, P. & Swamy, N. G. N. Derivatization of guar to sodium carboxymethyl hydroxypropyl derivative; characterization and evaluation. Pak. J. Pharm. Sci. 21, 40–44 (2008).
Sharma, B. R., Kumar, V . & Soni, P. L. Carbamoylethylation of guar gum. Carbohydr. Polym. 58, 449–453 (2004).
Surana, A., Mirza, Z. B. & Grover, P. D. Hydroxypropylation of guar gum and the effect of various process parameters on the yield and quality of hydroxypropyl guar gum. Res. Ind. 33, 49–54 (1988).
Grimm, M., Grabenwoger, M., Eybl, E., Moritz, A., Bock, P ., Muller, M. M. & Wolner, E. Improved biocompetability of biprosthetic heart valves by L-glutamic treatment. J. Card. Surg. 7, 58–64 (1992).
Raina, C. S., Singh, S., Bawa, A. S. & Saxena, D. C. Some characteristics of acetylated, cross-linked and dual modified Indian rice starches. Eur. Food Res. Technol. 223, 561–570 (2006).
Tattiyaku, J., Pradipasena, P. & Asavasaksakul, S. Taro colocasia esculenta (L.) Schott amylopectin structure and its effect on starch functional properties. Starch/Stärke 59, 342–347 (2007).
Xiao-yan, S., Qi-he, C., Hui, R., Guo-qing, H. & Qiong, X . Synthesis and paste properties of octenyl succinic anhydride modified early Indian rice starch. J. Zhejiang Univ. -Sci. B 7, 800–805 (2006).
Kwang, Y.L., Suyong, L . & Hyeon, G. L. Effect of the degree of enzymatic hydrolysis on the physicochemical properties and in vitro digestibility of rice starch. Food Sci. Biotechnol. 19, 1333–1340 (2010).
Yuan, R. C. & Thompson, D. B. Freeze thaw stability of three waxy maize starch pasted measured by centrifugation and calorimetry. Cereal Chem. 74, 571–573 (1998).
Sobolewska-Zieliñska, J. & Fortuna, T . Retrogradation of starches and maltodextrins of origin various. Acta Sci. Pol. Technol. Aliment. 9, 71–81 (2010).
Miles, M. J., Morris, V. J. & Ring, S. G. Some recent observations on the retrogradation of amylose. Carbohydr. Polym. 4, 73–77 (1984).
Jacobson, M., Obanni, M. & Bemiller, J. N. Retrogradation of starches from different botanical sources. Cereal Chem. 74, 511–518 (1997).
Ward, K. E. J., Hoseney, R. C. & Seib, P. A. Retrogradation of amylopectin from maize and wheat starches. Cereal Chem. 71, 150–155 (1994).
Atwell, W. A., Hood, L. F., Lineback, D. R., Varriano-Martson, E. & Zobel, H. F. The terminology and methodology associated with basic starch phenomena. Cereal Foods World 33, 306–311 (1988).
Lim, H. S., BeMiller, J. N. & Lim, S. T. Effect of dry heating with ionic gums at controlled pH on starch paste viscosity. Cereal Chem. 80, 198–202 (2003).
Wang, Q., Ellis, P. R. & Ross-Murphy, S. B. The stability of guar gum in an aqueous system under acidic conditions. Food Hydrocolloids 14, 129–134 (2000).
Alummoottil, N. J., Korappati, S., Moothandasseri, S. S., Revamma, R . & Subramoney, N.M. Gelatinisation properties of cassava starch in the presence of salts, acids and oxidising agents. Starch/Stärke 57, 547–555 (2005).
Amani, N. G., Kamenan, A., Rolland-Sabaté, A. & Colonna, P. Stability of yam starch gels during processing. Afr. J. Biotechnol. 4, 94–101 (2005).
Ogungbenle, H. N. Effect of chemical modification on starch of some legume flours. Pakistan J. Nutr. 6, 167–17 (2007).
Nur, A. & Purwiyatno, H. Gelatinization properties of white maize starch from three varieties of corn subject to oxidized and acetylated-oxidized modification. Int. Food Res. J. 17, 961–968 (2010).
Dzogbefia, V. P., Ofosu, G. A. & Oldham, J. H. Physicochemical and pasting properties of cassava starch extracted with the aid of pectin enzymes produced from Saccharomyces cerevisiae ATCC52712. Sci. Res. Essay 3, 406–409 (2008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hongbo, T., Yanping, L., Min, S. et al. Preparation and property of crosslinking guar gum. Polym J 44, 211–216 (2012). https://doi.org/10.1038/pj.2011.117
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2011.117
Keywords
This article is cited by
-
Influence of organoboron cross-linker and reservoir characteristics on filtration and reservoir residual of guar gum fracturing fluid in low-permeability shale gas reservoirs
Environmental Science and Pollution Research (2022)
-
Hydrogel Preparation Technologies: Relevance Kinetics, Thermodynamics and Scaling up Aspects
Journal of Polymers and the Environment (2019)
-
Oxidized Cross-Linked Guar Gum with Hydrophobic Groups: Structure, Properties and Removal of Reactive Blue-XBR in Simulated Water
Arabian Journal for Science and Engineering (2018)
-
Preparation and properties of partially hydrolyzed cross-linked guar gum
Polymer Bulletin (2013)