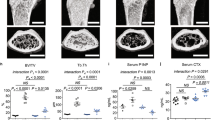
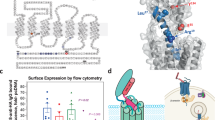
Abstract
Parathyroid hormone-related protein (PTHrP) is a critical regulator of bone resorption and augments osteolysis in skeletal malignancies. Here we report that the mature PTHrP1–36 hormone is processed by matrix metalloproteinases to yield a stable product, PTHrP1–17. PTHrP1–17 retains the ability to signal through PTH1R to induce calcium flux and ERK phosphorylation but not cyclic AMP production or CREB phosphorylation. Notably, PTHrP1–17 promotes osteoblast migration and mineralization in vitro, and systemic administration of PTHrP1–17 augments ectopic bone formation in vivo. Further, in contrast to PTHrP1–36, PTHrP1–17 does not affect osteoclast formation/function in vitro or in vivo. Finally, immunoprecipitation-mass spectrometry analyses using PTHrP1–17-specific antibodies establish that PTHrP1–17 is indeed generated by cancer cells. Thus, matrix metalloproteinase-directed processing of PTHrP disables the osteolytic functions of the mature hormone to promote osteogenesis, indicating important roles for this circuit in bone remodelling in normal and disease contexts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Miao D, He B, Jiang Y, Kobayashi T, Soroceanu MA, Zhao J et al. Osteoblast-derived PTHrP is a potent endogenous bone anabolic agent that modifies the therapeutic efficacy of administered PTH 1-34. J Clin Invest 2005; 115: 2402–2411.
Suva LJ, Winslow GA, Wettenhall RE, Hammonds RG, Moseley JM, Diefenbach-Jagger H et al. A parathyroid hormone-related protein implicated in malignant hypercalcemia: cloning and expression. Science 1987; 237: 893–896.
Martin TJ . Parathyroid hormone-related protein, its regulation of cartilage and bone development, and role in treating bone diseases. Physiol Rev 2016; 96: 831–871.
Guise TA, Yin JJ, Thomas RJ, Dallas M, Cui Y, Gillespie MT . Parathyroid hormone-related protein (PTHrP)-(1-139) isoform is efficiently secreted in vitro and enhances breast cancer metastasis to bone in vivo. Bone 2002; 30: 670–676.
Philbrick W . Parathyroid hormone-related protein: Gene structure, biosynthesis, metabolism, and regulation. In: Bilezikian JP, Marcus R, Levine M (eds). The Parathyroids: Basic and Clinical Concepts. 2nd edn. Academic Press: San Diego, CA, USA, 2001, p 881.
Bilezikian JP, Marcus R, Levine M, Marcocci C, Silverberg SJ, Potts J (eds). The Parathyroids: Basic and Clinical Concepts. 3rd edn. Academic Press: San Diego, CA, USA, 2015, p 946.
Juppner H, Abou-Samra AB, Freeman M, Kong XF, Schipani E, Richards J et al. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science 1991; 254: 1024–1026.
Orloff JJ, Reddy D, de Papp AE, Yang KH, Soifer NE, Stewart AF . Parathyroid hormone-related protein as a prohormone: posttranslational processing and receptor interactions. Endocr Rev 1994; 15: 40–60.
Park HJ, Baek K, Baek JH, Kim HR . The cooperation of CREB and NFAT is required for PTHrP-induced RANKL expression in mouse osteoblastic cells. J Cell Physiol 2015; 230: 667–679.
Fukushima H, Jimi E, Kajiya H, Motokawa W, Okabe K . Parathyroid-hormone-related protein induces expression of receptor activator of NF-{kappa}B ligand in human periodontal ligament cells via a cAMP/protein kinase A-independent pathway. J Dent Res 2005; 84: 329–334.
Ma YL, Cain RL, Halladay DL, Yang X, Zeng Q, Miles RR et al. Catabolic effects of continuous human PTH (1–38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and gene-associated bone formation. Endocrinology 2001; 142: 4047–4054.
Miao D, Li J, Xue Y, Su H, Karaplis AC, Goltzman D . Parathyroid hormone-related peptide is required for increased trabecular bone volume in parathyroid hormone-null mice. Endocrinology 2004; 145: 3554–3562.
Stewart AF . PTHrP(1-36) as a skeletal anabolic agent for the treatment of osteoporosis. Bone 1996; 19: 303–306.
McCauley LK, Martin TJ . Twenty-five years of PTHrP progress: from cancer hormone to multifunctional cytokine. J Bone Miner Res 2012; 27: 1231–1239.
Cramer SD, Chen Z, Peehl DM . Prostate specific antigen cleaves parathyroid hormone-related protein in the PTH-like domain: inactivation of PTHrP-stimulated cAMP accumulation in mouse osteoblasts. J Urol 1996; 156 (2 Pt 1): 526–531.
Philbrick WM, Wysolmerski JJ, Galbraith S, Holt E, Orloff JJ, Yang KH et al. Defining the roles of parathyroid hormone-related protein in normal physiology. Physiol Rev 1996; 76: 127–173.
Martin TJ . Osteoblast-derived PTHrP is a physiological regulator of bone formation. J Clin Invest 2005; 115: 2322–2324.
Ruchon AF, Marcinkiewicz M, Ellefsen K, Basak A, Aubin J, Crine P et al. Cellular localization of neprilysin in mouse bone tissue and putative role in hydrolysis of osteogenic peptides. J Bone Miner Res 2000; 15: 1266–1274.
Lopez-Otin C, Matrisian LM . Emerging roles of proteases in tumour suppression. Nat Rev Cancer 2007; 7: 800–808.
Krane SM, Inada M . Matrix metalloproteinases and bone. Bone 2008; 43: 7–18.
Lynch CC . Matrix metalloproteinases as master regulators of the vicious cycle of bone metastasis. Bone 2010; 48: 44–53.
Lopez-Otin C, Overall CM . Protease degradomics: a new challenge for proteomics. Nat Rev Mol Cell Biol 2002; 3: 509–519.
Lynch CC . Matrix metalloproteinases as master regulators of the vicious cycle of bone metastasis. Bone 2011; 48: 44–53.
Lynch CC, Hikosaka A, Acuff HB, Martin MD, Kawai N, Singh RK et al. MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell 2005; 7: 485–496.
Winding B, NicAmhlaoibh R, Misander H, Hoegh-Andersen P, Andersen TL, Holst-Hansen C et al. Synthetic matrix metalloproteinase inhibitors inhibit growth of established breast cancer osteolytic lesions and prolong survival in mice. Clin Cancer Res 2002; 8: 1932–1939.
Bonfil RD, Sabbota A, Nabha S, Bernardo MM, Dong Z, Meng H et al. Inhibition of human prostate cancer growth, osteolysis and angiogenesis in a bone metastasis model by a novel mechanism-based selective gelatinase inhibitor. Int J Cancer 2006; 118: 2721–2726.
Croucher PI, McDonald MM, Martin TJ . Bone metastasis: the importance of the neighbourhood. Nat Rev Cancer 2016; 16: 373–386.
Guise TA . Parathyroid hormone-related protein and bone metastases. Cancer 1997; 80 (8 Suppl): 1572–1580.
Mundy GR . Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2002; 2: 584–593.
Thiolloy S, Edwards JR, Fingleton B, Rifkin DB, Matrisian LM, Lynch CC . An osteoblast-derived proteinase controls tumor cell survival via TGF-beta activation in the bone microenvironment. PLoS One 2012; 7: e29862.
Cupp ME, Nayak SK, Adem AS, Thomsen WJ . Parathyroid hormone (PTH) and PTH-related peptide domains contributing to activation of different PTH receptor-mediated signaling pathways. J Pharmacol Exp Ther 2013; 345: 404–418.
Jilka RL, Weinstein RS, Bellido T, Parfitt AM, Manolagas SC . Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res 1998; 13: 793–802.
Esbrit P, Alcaraz MJ . Current perspectives on parathyroid hormone (PTH) and PTH-related protein (PTHrP) as bone anabolic therapies. Biochem Pharmacol 2013; 85: 1417–1423.
Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 1999; 99: 81–92.
Pettway GJ, Schneider A, Koh AJ, Widjaja E, Morris MD, Meganck JA et al. Anabolic actions of PTH (1-34): use of a novel tissue engineering model to investigate temporal effects on bone. Bone 2005; 36: 959–970.
Yates AJ, Gutierrez GE, Smolens P, Travis PS, Katz MS, Aufdemorte TB et al. Effects of a synthetic peptide of a parathyroid hormone-related protein on calcium homeostasis, renal tubular calcium reabsorption, and bone metabolism in vivo and in vitro in rodents. J Clin Invest 1988; 81: 932–938.
Anderson NL, Anderson NG, Haines LR, Hardie DB, Olafson RW, Pearson TW . Mass spectrometric quantitation of peptides and proteins using stable isotope standards and capture by anti-peptide antibodies (SISCAPA). J Proteome Res 2004; 3: 235–244.
Katafuchi T, Esterhazy D, Lemoff A, Ding X, Sondhi V, Kliewer SA et al. Detection of FGF15 in plasma by stable isotope standards and capture by anti-peptide antibodies and targeted mass spectrometry. Cell Metab 2015; 21: 898–904.
Yoon H, Blaber SI, Li W, Scarisbrick IA, Blaber M . Activation profiles of human kallikrein-related peptidases by matrix metalloproteinases. Biol Chem 2013; 394: 137–147.
Pezzato E, Sartor L, Dell'Aica I, Dittadi R, Gion M, Belluco C et al. Prostate carcinoma and green tea: PSA-triggered basement membrane degradation and MMP-2 activation are inhibited by (-)epigallocatechin-3-gallate. Int J Cancer 2004; 112: 787–792.
Kawashima-Ohya Y, Satakeda H, Kuruta Y, Kawamoto T, Yan W, Akagawa Y et al. Effects of parathyroid hormone (PTH) and PTH-related peptide on expressions of matrix metalloproteinase-2, -3, and -9 in growth plate chondrocyte cultures. Endocrinology 1998; 139: 2120–2127.
Ibaragi S, Shimo T, Iwamoto M, Hassan NM, Kodama S, Isowa S et al. Parathyroid hormone-related peptide regulates matrix metalloproteinase-13 gene expression in bone metastatic breast cancer cells. Anticancer Res 2010; 30: 5029–5036.
Washam CL, Byrum SD, Leitzel K, Ali SM, Tackett AJ, Gaddy D et al. Identification of PTHrP(12-48) as a plasma biomarker associated with breast cancer bone metastasis. Cancer Epidemiol Biomarkers Prev 2013; 22: 972–983.
Amizuka N, Henderson JE, White JH, Karaplis AC, Goltzman D, Sasaki T et al. Recent studies on the biological action of parathyroid hormone (PTH)-related peptide (PTHrP) and PTH/PTHrP receptor in cartilage and bone. Histol Histopathol 2000; 15: 957–970.
Cuthbertson RM, Kemp BE, Barden JA . Structure study of osteostatin PTHrP[Thr107](107-139). Biochim Biophys Acta 1999; 1432: 64–72.
Valin A, Garcia-Ocana A, De Miguel F, Sarasa JL, Esbrit P . Antiproliferative effect of the C-terminal fragments of parathyroid hormone-related protein, PTHrP-(107-111) and (107-139), on osteoblastic osteosarcoma cells. J Cell Physiol 1997; 170: 209–215.
Garcia-Martin A, Ardura JA, Maycas M, Lozano D, Lopez-Herradon A, Portal-Nunez S et al. Functional roles of the nuclear localization signal of parathyroid hormone-related protein (PTHrP) in osteoblastic cells. Mol Endocrinol 2014; 28: 925–934.
Lai CF, Chaudhary L, Fausto A, Halstead LR, Ory DS, Avioli LV et al. Erk is essential for growth, differentiation, integrin expression, and cell function in human osteoblastic cells. J Biol Chem 2001; 276: 14443–14450.
Azarani A, Goltzman D, Orlowski J . Structurally diverse N-terminal peptides of parathyroid hormone (PTH) and PTH-related peptide (PTHRP) inhibit the Na+/H+ exchanger NHE3 isoform by binding to the PTH/PTHRP receptor type I and activating distinct signaling pathways. J Biol Chem 1996; 271: 14931–14936.
Luck MD, Carter PH, Gardella TJ . The (1-14) fragment of parathyroid hormone (PTH) activates intact and amino-terminally truncated PTH-1 receptors. Mol Endocrinol 1999; 13: 670–680.
Takuwa Y, Ohue Y, Takuwa N, Yamashita K . Endothelin-1 activates phospholipase C and mobilizes Ca2+ from extra- and intracellular pools in osteoblastic cells. Am J Physiol 1989; 257 (6 Pt 1): E797–E803.
Schluter KD, Katzer C, Piper HM . A N-terminal PTHrP peptide fragment void of a PTH/PTHrP-receptor binding domain activates cardiac ET(A) receptors. Br J Pharmacol 2001; 132: 427–432.
Frieling JS, Basanta D, Lynch CC . Current and emerging therapies for bone metastatic castration-resistant prostate cancer. Cancer Control: Journal of the Moffitt Cancer Center 2015; 22: 109–120.
Mak IW, Turcotte RE, Ghert M . Parathyroid hormone-related protein (PTHrP) modulates adhesion, migration and invasion in bone tumor cells. Bone 2013; 55: 198–207.
Hodde JP, Suckow MA, Wolter WR, Hiles MC . Small intestinal submucosa does not promote PAIII tumor growth in Lobund-Wistar rats. J Surg Res 2004; 120: 189–194.
Wu TT, Sikes RA, Cui Q, Thalmann GN, Kao C, Murphy CF et al. Establishing human prostate cancer cell xenografts in bone: induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. Int J Cancer 1998; 77: 887–894.
Helfrich MH, Ralston S . Bone Research Protocols. Humana Press: Totowa, NJ, 2003, pxiv, 448p.
Remily-Wood ER, Liu RZ, Xiang Y, Chen Y, Thomas CE, Rajyaguru N et al. A database of reaction monitoring mass spectrometry assays for elucidating therapeutic response in cancer. Proteomics Clin Appl 2011; 5: 383–396.
Gallien S, Duriez E, Crone C, Kellmann M, Moehring T, Domon B . Targeted proteomic quantification on quadrupole-orbitrap mass spectrometer. Mol Cell Proteomics 2012; 11: 1709–1723.
MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B et al. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 2010; 26: 966–968.
Zhao W, Byrne MH, Boyce BF, Krane SM . Bone resorption induced by parathyroid hormone is strikingly diminished in collagenase-resistant mutant mice. J Clin Invest 1999; 103: 517–524.
Cook LM, Araujo A, Pow-Sang JM, Budzevich MM, Basanta D, Lynch CC . Predictive computational modeling to define effective treatment strategies for bone metastatic prostate cancer. Sci Rep 2016; 6: 29384.
Mohammad KS, Chirgwin JM, Guise TA . Assessing new bone formation in neonatal calvarial organ cultures. Methods Mol Biol 2008; 455: 37–50.
Datta NS, Pettway GJ, Chen C, Koh AJ, McCauley LK . Cyclin D1 as a target for the proliferative effects of PTH and PTHrP in early osteoblastic cells. J Bone Miner Res 2007; 22: 951–964.
Acknowledgements
This work was supported by R01-CA143094 (CCL), the State of Florida Bankhead Coley Program grant 5BC-O1 (CCL) and by a Miles for Moffitt Foundation Award. This work was also supported in part by the Core Facilities of the Moffitt Cancer Center Grant P30-CA076292. The authors thank Joe Johnson (Moffitt Analytical Microscopy), Robert Sprung (Moffitt Proteomics) and Gordon Whiteley (SAIC-Frederick, NCI-Frederick) for their helpful suggestions and expertise, and Drs John Cleveland and Srikumar Chellappan for critical review of this study.
Author contributions
CCL and JSF designed all of the biological experiments. JSF conducted the majority of the in vitro and in vivo experimental assays and collection of data. GS contributed to osteoclast in vitro and in vivo analyses. RGS was responsible for the generation and initial characterization of PTHrP1–17 specific antibodies. SA assisted with the analysis of real time PCR data and statistical analyses. MB assisted with μCT experiments and analyses. JK and VI designed, performed and analysed the proteomic experiments. JSF, JK, GS, and CCL wrote, edited and proofread the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Frieling, J., Shay, G., Izumi, V. et al. Matrix metalloproteinase processing of PTHrP yields a selective regulator of osteogenesis, PTHrP1–17. Oncogene 36, 4498–4507 (2017). https://doi.org/10.1038/onc.2017.70
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2017.70