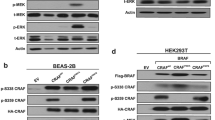
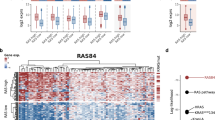
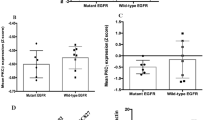
Abstract
Recent studies suggest that the presence of a KRAS mutation may be insufficient for defining a clinically homogenous molecular group, as many KRAS mutant tumors lose reliance on K-Ras for survival. Identifying pathways that support K-Ras dependency may define clinically relevant KRAS subgroups and lead to the identification of new drug targets. We have analyzed a panel of 17 KRAS mutant lung cancer cell lines classified as K-Ras-dependent or -independent for co-dependency on protein kinase C δ (PKCδ). We show that functional dependency on K-Ras and PKCδ co-segregate, and that dependency correlates with a more epithelial-like phenotype. Furthermore, we show that the pro-apoptotic and pro-tumorigenic functions of PKCδ also segregate based on K-Ras dependency, as K-Ras-independent cells are more sensitive to topoisomerase inhibitors, and depletion of PKCδ in this subgroup suppresses apoptosis through increased activation of extracellular signal-regulated kinase (ERK). In contrast, K-Ras-dependent lung cancer cells are largely insensitive to topoisomerase inhibitors, and depletion of PKCδ can increase apoptosis and decrease activation of ERK in this subgroup. We have previously shown that nuclear translocation of PKCδ is necessary and sufficient for pro-apoptotic signaling. Our current studies show that K-Ras-dependent cells are refractive to PKCδ-driven apoptosis. Analysis of this subgroup showed increased PKCδ expression and an increase in the nuclear:cytoplasmic ratio of PKCδ. In addition, targeting PKCδ to the nucleus induces apoptosis in K-Ras-independent, but not K-Ras-dependent non-small-cell lung cancer (NSCLC) cells. Our studies provide tools for identification of the subset of patients with KRAS mutant tumors most amenable to targeting of the K-Ras pathway, and identify PKCδ as a potential target in this tumor population. These subgroups are likely to be of clinical relevance, as high PKCδ expression correlates with increased overall survival and a more epithelial tumor phenotype in patients with KRAS mutant lung adenocarcinomas.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Beau-Faller M, Legrain M, Voegeli AC, Guerin E, Lavaux T, Ruppert AM et al. Detection of K-Ras mutations in tumour samples of patients with non-small cell lung cancer using PNA-mediated PCR clamping. Br J Cancer 2009; 100: 985–992.
Singh A, Greninger P, Rhodes D, Koopman L, Violette S, Bardeesy N et al. A gene expression signature associated with ‘K-Ras addiction’ reveals regulators of EMT and tumor cell survival. Cancer Cell 2009; 15: 489–500.
Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med 2011; 17: 500–503.
McCormick F . KRAS as a therapeutic target. Clin Cancer Res 2015; 21: 1797–1801.
Janne PA, Shaw AT, Pereira JR, Jeannin G, Vansteenkiste J, Barrios C et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol 2013; 14: 38–47.
Xia S, Chen Z, Forman LW, Faller DV . PKCdelta survival signaling in cells containing an activated p21Ras protein requires PDK1. Cell Signal 2009; 21: 502–508.
Xia S, Forman LW, Faller DV . Protein kinase C delta is required for survival of cells expressing activated p21RAS. J Biol Chem 2007; 282: 13199–13210.
Symonds JM, Ohm AM, Tan AC, Reyland ME . PKCdelta regulates integrin alphaVbeta3 expression and transformed growth of K-ras dependent lung cancer cells. Oncotarget 2016; 7: 17905–17919.
Symonds JM, Ohm AM, Carter CJ, Heasley LE, Boyle TA, Franklin WA et al. Protein kinase C delta is a downstream effector of oncogenic K-ras in lung tumors. Cancer Res 2011; 71: 2087–2097.
Reyland ME, Jones DN . Multifunctional roles of PKCdelta: opportunities for targeted therapy in human disease. Pharmacol Ther 2016; 165: 1–13.
Garg R, Benedetti LG, Abera MB, Wang H, Abba M, Kazanietz MG . Protein kinase C and cancer: what we know and what we do not. Oncogene 2014; 33: 5225–5237.
Nguyen HM, Reyland ME, Barlow LA . Mechanisms of taste bud cell loss after head and neck irradiation. J Neurosci 2012; 32: 3474–3484.
Allen-Petersen BL, Miller MR, Neville MC, Anderson SM, Nakayama KI, Reyland ME . Loss of protein kinase C delta alters mammary gland development and apoptosis. Cell Death Dis 2010; 1: e17.
Humphries MJ, Limesand KH, Schneider JC, Nakayama KI, Anderson SM, Reyland ME . Suppression of apoptosis in the protein kinase C delta null mouse in vivo. J Biol Chem 2006; 281: 9728–9737.
Allen-Petersen BL, Carter CJ, Ohm AM, Reyland ME . Protein kinase C delta is required for ErbB2-driven mammary gland tumorigenesis and negatively correlates with prognosis in human breast cancer. Oncogene 2014; 33: 1306–1315.
Mauro LV, Grossoni VC, Urtreger AJ, Yang C, Colombo LL, Morandi A et al. PKC Delta (PKCdelta) promotes tumoral progression of human ductal pancreatic cancer. Pancreas 2010; 39: e31–e41.
Jackson D, Zheng Y, Lyo D, Shen Y, Nakayama K, Nakayama KI et al. Suppression of cell migration by protein kinase C delta. Oncogene 2005; 24: 3067–3072.
Kho DH, Bae JA, Lee JH, Cho HJ, Cho SH, Lee JH et al. KITENIN recruits dishevelled/PKC delta to form a functional complex and controls the migration and invasiveness of colorectal cancer cells. Gut 2009; 58: 509–519.
Paugh BS, Paugh SW, Bryan L, Kapitonov D, Wilczynska KM, Gopalan SM et al. EGF regulates plasminogen activator inhibitor-1 (PAI-1) by a pathway involving c-Src, PKCdelta, and sphingosine kinase 1 in glioblastoma cells. FASEB J 2008; 22: 455–465.
Kharait S, Dhir R, Lauffenburger D, Wells A . Protein kinase C delta signaling downstream of the EGF receptor mediates migration and invasiveness of prostate cancer cells. Biochem Biophys Res Commun 2006; 343: 848–856.
Bailey TA, Luan H, Tom E, Bielecki TA, Mohapatra B, Ahmad G et al. A kinase inhibitor screen reveals protein kinase C-dependent endocytic recycling of ErbB2 in breast cancer cells. J Biol Chem 2014; 289: 30443–30458.
Hu CT, Cheng CC, Pan SM, Wu JR, Wu WS . PKC mediates fluctuant ERK-paxillin signaling for hepatocyte growth factor-induced migration of hepatoma cell HepG2. Cell Signal 2013; 25: 1457–1467.
Park M, Kim WK, Song M, Park M, Kim H, Nam HJ et al. Protein kinase C-delta-mediated recycling of active KIT in colon cancer. Clin Cancer Res 2013; 19: 4961–4971.
Reyland ME, Barzen KA, Anderson SM, Quissell DO, Matassa AA . Activation of PKC is sufficient to induce an apoptotic program in salivary gland acinar cells. Cell Death Differ 2000; 7: 1200–1209.
Matassa AA, Carpenter L, Biden TJ, Humphries MJ, Reyland ME . PKCdelta is required for mitochondrial-dependent apoptosis in salivary epithelial cells. J Biol Chem 2001; 276: 29719–29728.
Yoshida K . Nuclear trafficking of pro-apoptotic kinases in response to DNA damage. Trends Mol Med 2008; 14: 305–313.
Mogi A, Kuwano H . TP53 mutations in nonsmall cell lung cancer. J Biomed Biotechnol 2011; 2011: 583929.
Osaki S, Nakanishi Y, Takayama K, Pei XH, Ueno H, Hara N . Alteration of drug chemosensitivity caused by the adenovirus-mediated transfer of the wild-type p53 gene in human lung cancer cells. Cancer Gene Ther 2000; 7: 300–307.
Kolb RH, Greer PM, Cao PT, Cowan KH, Yan Y . ERK1/2 signaling plays an important role in topoisomerase II poison-induced G2/M checkpoint activation. PloS One 2012; 7: e50281.
DeVries-Seimon TA, Ohm AM, Humphries MJ, Reyland ME . Induction of apoptosis is driven by nuclear retention of protein kinase C delta. J Biol Chem 2007; 282: 22307–22314.
Adwan TS, Ohm AM, Jones DN, Humphries MJ, Reyland ME . Regulated binding of importin-alpha to protein kinase Cdelta in response to apoptotic signals facilitates nuclear import. J Biol Chem 2011; 286: 35716–35724.
Humphries MJ, Ohm AM, Schaack J, Adwan TS, Reyland ME . Tyrosine phosphorylation regulates nuclear translocation of PKCdelta. Oncogene 2008; 27: 3045–3053.
Wie SM, Adwan TS, DeGregori J, Anderson SM, Reyland ME . Inhibiting tyrosine phosphorylation of protein kinase Cdelta (PKCdelta) protects the salivary gland from radiation damage. J Biol Chem 2014; 289: 10900–10908.
DeVries TA, Neville MC, Reyland ME . Nuclear import of PKCdelta is required for apoptosis: identification of a novel nuclear import sequence. EMBO J 2002; 21: 6050–6060.
Riely GJ, Marks J, Pao W . KRAS mutations in non-small cell lung cancer. Proc Am Thorac Soc 2009; 6: 201–205.
Pagliarini R, Shao W, Sellers WR . Oncogene addiction: pathways of therapeutic response, resistance, and road maps toward a cure. EMBO Rep 2015; 16: 280–296.
LaGory EL, Sitailo LA, Denning MF . The protein kinase C delta catalytic fragment is critical for maintenance of the G2/M DNA damage checkpoint. J Biol Chem 2010; 285: 1879–1887.
Ueda Y, Hirai S, Osada S, Suzuki A, Mizuno K, Ohno S . Protein kinase C activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J Biol Chem 1996; 271: 23512–23519.
Koo KH, Jeong WJ, Cho YH, Park JC, Min DS, Choi KY . K-Ras stabilization by estrogen via PKC delta is involved in endometrial tumorigenesis. Oncotarget 2015; 6: 21328–21340.
Antal CE, Hudson AM, Kang E, Zanca C, Wirth C, Stephenson NL et al. Cancer-associated protein kinase C mutations reveal kinase's role as tumor suppressor. Cell 2015; 160: 489–502.
D'Costa AM, Robinson JK, Maududi T, Chaturvedi V, Nickoloff BJ, Denning MF . The proapoptotic tumor suppressor protein kinase C-delta is lost in human squamous cell carcinomas. Oncogene 2006; 25: 378–386.
Reno EM, Haughian JM, Dimitrova IK, Jackson TA, Shroyer KR, Bradford AP . Analysis of protein kinase C delta (PKC delta) expression in endometrial tumors. Hum Pathol 2008; 39: 21–29.
Imielinski M, Berger AH, Hammerman PS, Hernandez B, Pugh TJ, Hodis E et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell 2012; 150: 1107–1120.
Young A, Lou D, McCormick F . Oncogenic and wild-type Ras play divergent roles in the regulation of mitogen-activated protein kinase signaling. Cancer Discov 2013; 3: 112–123.
Cai Q, Li J, Gao T, Xie J, Evers BM . Protein kinase C delta negatively regulates hedgehog signaling by inhibition of Gli1 activity. J Biol Chem 2009; 284: 2150–2158.
Zhu T, Tsuji T, Chen C . Roles of PKC isoforms in the induction of apoptosis elicited by aberrant Ras. Oncogene 2010; 29: 1050–1061.
Luo J, Emanuele MJ, Li D, Creighton CJ, Schlabach MR, Westbrook TF et al. A genome-wide RNAi screen identifies multiple synthetic lethal interactions with the Ras oncogene. Cell 2009; 137: 835–848.
Wilson FH, Johannessen CM, Piccioni F, Tamayo P, Kim JW, Van Allen EM et al. A functional landscape of resistance to ALK inhibition in lung cancer. Cancer Cell 2015; 27: 397–408.
Prior IA, Lewis PD, Mattos C . A comprehensive survey of Ras mutations in cancer. Cancer Res 2012; 72: 2457–2467.
Singh A, Settleman J . Oncogenic K-ras ‘addiction’ and synthetic lethality. Cell Cycle 2009; 8: 2676–2677.
Acknowledgements
We gratefully acknowledge the intellectual contributions of Dr Ross Camidge. This work was supported by a United Against Lung Cancer research award, a pilot grant from NIH Lung SPORE Grant P50 CA58187 and R01DE015648 to MER.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Ohm, A., Tan, AC., Heasley, L. et al. Co-dependency of PKCδ and K-Ras: inverse association with cytotoxic drug sensitivity in KRAS mutant lung cancer. Oncogene 36, 4370–4378 (2017). https://doi.org/10.1038/onc.2017.27
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2017.27